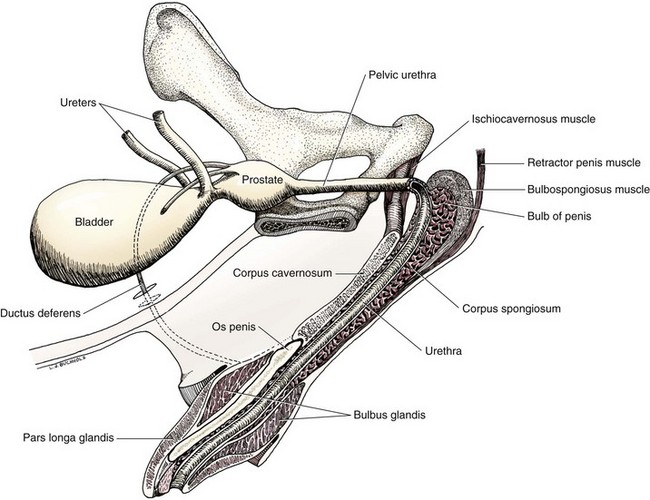
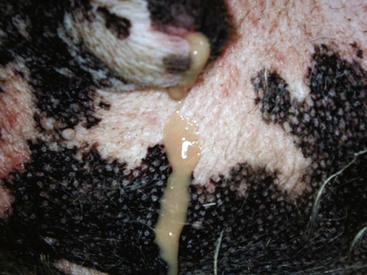
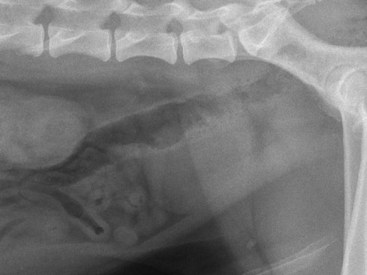
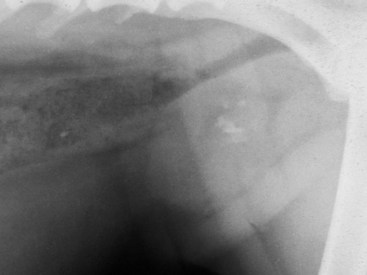
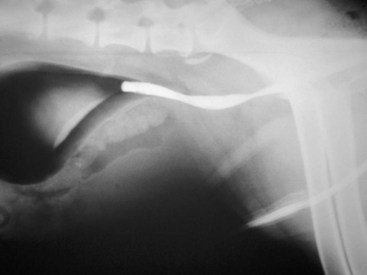
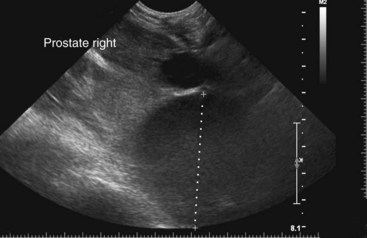
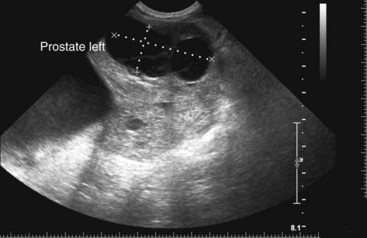
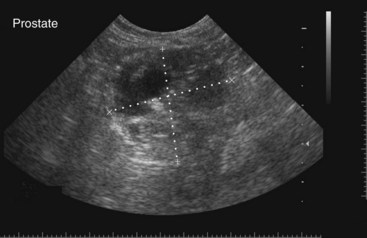
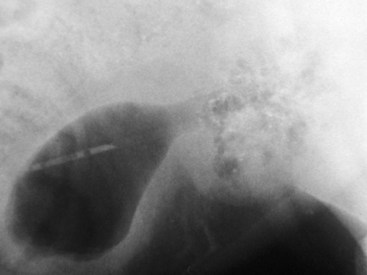
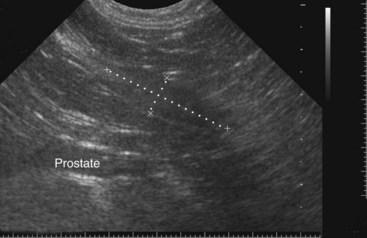
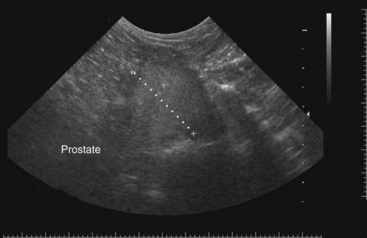
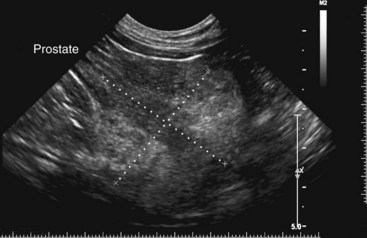
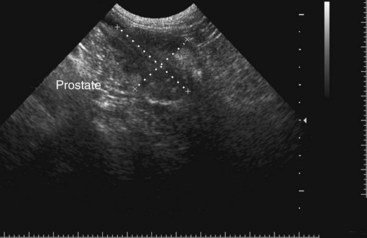
Chapter 113 The canine prostate gland is a bilobed structure that completely encircles the proximal urethra immediately caudal to the bladder (Figure 113-1). It is the sole accessory sex organ of male dogs.28 Prostatic tissue is also present in cats as a smaller structure that only partly encircles the urethra. The prostate gland has an endodermal origin and arises from the pelvic urethral epithelium. A series of symmetric buds, the prostatic tubules, appear as solid epithelial projections from the urethra at about the sixth week of gestation in the dog75 and grow into the surrounding mesenchyme. The glandular epithelium develops from these endodermal cells while the mesenchymal tissue differentiates into the stroma and smooth muscle of the prostate.67 Much of the smooth muscle in the prostate originates from the prostatic urethra,83 which develops from the pelvic part of the urogenital sinus. The adult gland therefore represents a composite collection of mesenchymal, urethral, and Wolffian duct tissue with glandular and nonglandular components bound within a common capsule. The size and weight of the prostate gland varies with age, breed, and body weight.24 After sexual maturity, the gland undergoes rapid enlargement to its normal adult weight and thereafter continues to enlarge slowly. Maintenance of the normal prostate gland is androgen driven, and involution occurs after castration and, in some cases, with senility. Various authors have proposed a range for the normal prostate gland to body weight ratio in mature adult dogs of 0.64 to 0.96 g per kilogram body weight.8,9,24 Ratios greater than this range were invariably associated with histologic abnormality, although in some breeds, notably Scottish terriers, the range has been reported to be significantly greater.72 A correlation between age or body weight and prostate length, depth on longitudinal and transverse sections, and width as estimated on ultrasound examination has been described.2,82 The craniocaudal position of the gland is age dependent, and it remains abdominal until the urachal vestige breaks down at 2 months of age, at which time it occupies a pelvic position as a small swelling around the proximal urethra. At puberty, the gland enlarges and migrates to occupy a partially abdominal position. This development is most marked between 20 and 32 weeks of age.53 During adult life, the gland continues to undergo hyperplastic enlargement and migrates further cranially42,43 with half of the gland reported to be abdominal by 4 years of age. In some chondrodystrophoid dogs, the gland is intra-abdominal from birth. Eventually, the entire gland becomes abdominal; this process is reported to not be complete until 10 years of age,28 but in most dogs, abdominal migration occurs long before then. The adult prostate gland is found in the caudal abdomen bound dorsally by the ventral surface of the rectum and laterally by the levator ani muscles and supported ventrally by the pubic symphysis and abdominal wall. The prostate is separated from the rectum by the folds of the peritoneum filling the rectogenital space; therefore, although its dorsal surface has a peritoneal covering, its ventral aspect is retroperitoneal. A fibrous band is found between the caudal third of the dorsal prostatic surface and the rectum.41 The gland is bilobed with a dorsal and ventral midline sulcus separating the right and left lobes. The limits of the lobes are rendered less distinct by virtue of being enclosed in a fibromuscular capsule; smooth muscle fibers extend from the bladder wall to the dorsal surface of the capsule.28 The overall transverse shape of the lobes is ovoid with the urethra positioned closer to the dorsal surface of the gland. The craniodorsal surfaces of the prostate are perforated by the vasa deferentia, which enter the gland bilaterally before opening into the urethra via two slits on each side of the colliculis seminalis. The two lobes of the prostate gland have an abundant independent vascular supply from the prostatic vessels. The prostatic arteries branch from the internal pudendal vessels, or occasionally more proximally from the umbilical arteries, at the level of S2-S3. They supply the arteries of the ductus deferens, which in turn give rise to the caudal vesical arteries and then the caudal rectal vessels. There are significant anastomoses between the prostatic arteries and the urethral and cranial and caudal rectal arteries.28 The prostatic arteries divide into cranial, middle, and caudal branches before distributing across the dorsolateral surfaces, becoming subcapsular arteries, and supplying the glandular tissue.49,90 The vascular zones of the prostate have been described as capsular, parenchymal, and urethral.90 The parenchyma is supplied by trabecular branches. Two types of trabecular vessels are recognized: direct and branched. The periacinary capillaries are fenestrated and form a circular network pattern. The prostatic section of the urethra is supplied by an independent branch of the prostatic arteries; the cavernous tissue immediately surrounding the prostatic urethra, however, is supplied by the artery of the bulb of the penis. Venous drainage from the gland is via the prostatic and urethral veins into the internal iliac vein. The prostatic urethra is drained by the prostatic veins, the vein of the urethral bulb, and the ventral prostatic veins. Lymphatic drainage takes place via a network on the surface of the gland, which then drains to the medial iliac and hypogastric chain of nodes. The autonomic nerve supply to the gland is via the hypogastric and pelvic nerves. The hypogastric nerve follows the route of the artery of the deferent duct, and the pelvic nerve follows the prostatic artery as far as the lateral surface of the rectum, where it joins branches of the hypogastric nerve to form the pelvic plexus. The gland is supplied from this via the prostatic plexus.28 The activity of the secretory epithelium responsible for fluid excretion is controlled via cholinergic postganglionic fibers from the hypogastric (sympathetic) nerve96 that are also found in stromal tissue.73 Smooth muscle contractions responsible for dynamic fluid movement are regulated by adrenergic postganglionic fibers from the hypogastric nerve. The parasympathetic supply from the pelvic nerve has been reported to increase the rate of glandular secretion,28 but others report that its role is other than fluid secretion.86,96 The stromal tissue of the prostate contains rich noradrenergic innervation that controls prostatic smooth muscle tone. A range of neurotransmitters, including adenine nucleotides and nucleosides, nitric oxide, opioids, neuropeptide Y, and vasoactive intestinal peptide, may act as inhibitory cotransmitters or regulators of autonomic function in stromal tissue.73 The prostate gland comprises secretory epithelial tissue contained within a stromal capsule of fibrous, elastic, and smooth muscle tissue. The epithelial tissue is subdivided into distinct lobules by smooth muscle septa that extend throughout the gland from the capsule to the urethra. The tissue within the lobules is organized into tubuloalveolar glands that converge with draining ducts that empty into the urethra. The canine prostate lacks the zonal differentiation seen in the human gland, containing more prominent acini and less stromal contribution.58 Prostates from sexually immature dogs are characterized by a branching ductular system; the acinar portions of the gland are not developed, have few lumina, and show no evidence of secretory function.24 Immature prostatic epithelium is characterized by a cuboidal to flattened basophilic epithelium with a high nuclear-to-cytoplasmic ratio. The amount of connective tissue is relatively higher in immature glands than in fully developed glands. This stroma is composed predominantly of fibrous connective tissue with smooth muscle in the outer capsule and in major trabeculae radiating into the gland. The first histologic signs of activity appear at about 4 months of age when the acinar cells become periodic acid–Schiff positive, indicating secretory activity: evidence of eosinophilic material in the acinar cell cytoplasm or acinar lumen53 indicates onset of secretory activity. Mature prostates are characterized by compound tubuloalveolar glands, which radiate from their duct openings into the urethra. The acinar portion of the gland is dilated to form an alveolar structure and contains primary and secondary infoldings of secretory epithelium that project into the alveolar lumina. Mature prostatic epithelia are characterized by columnar epithelial cells with basal nuclei and abundant eosinophilic apical cytoplasm. Prostatic secretion is characterized by a deeply eosinophilic secretion dilating the acini but without compression of adjacent acini. Between 23 and 89 weeks of age, secretion increases with age.24 In mature prostates, two patterns of acinar dilatation have been recognized: (1) simple dilatation with many dilated acini with or without luminal eosinophilic secretion that does not compress the adjacent acini (acini are lined by a flattened basophilic epithelium with a high nuclear-to-cytoplasmic ratio) and (2) focal glandular ectasia with focal dilatation of a few acini with eosinophilic content, sharply demarcated, with compression of the adjacent prostatic parenchyma. Acini are lined by a columnar epithelium with basal nuclei and abundant eosinophilic apical cytoplasm. The alveoli are separated by a dense stroma that also contains smooth muscle cells. Smooth muscle septa are continuous with the smooth muscle and fibrous tissue located in the periurethral area.24 Secretion is an androgen-mediated function of the prostate that ceases rapidly after castration. The fluid secreted by the prostate gland and released into the ejaculate is thought to promote sperm motility and viability, although its precise function remains obscure. The normal pH of the secretion in the dog is 6.1 to 6.5; this is considerably more acidic than that in men, in which its alkaline nature (7.3) is considered necessary to neutralize the acidic vaginal pH.30 Sodium concentrations in the prostatic fluid of anesthetized dogs were similar to that of plasma, suggesting passive movement; potassium and chloride concentrations were higher than plasma concentrations, indicating active transport into the fluid.87 The volume of the secretion in the ejaculate varies considerably with age. Secretion normally begins at puberty, increasing to a maximum of approximately 20 mL in Beagles by the age of 4 years and then declines to minimal levels by 8 to 9 years of age.14 The contribution from prostatic secretion is widely considered to be contained in the third fraction of the ejaculate, but some studies have also reported prostatic contributions in the first and third fractions.27 Prostatic secretions have been shown to contain high concentrations of zinc that predictably are found localized primarily in the nucleolus, nuclear chromatin, and secretory granules of the epithelial cells.17 The true role of zinc in prostatic secretions is unclear. The presence of zinc is known to have an antibacterial effect, but there was no correlation between zinc concentrations and resistance to infection in one study.21 Unlike the case with the human prostate, inflammatory diseases do not appear to cause a change in the concentration of zinc in canine ejaculate and many aspects of secretory function, including pH; specific gravity; and copper, iron, and magnesium content also appear to be unchanged;12 castration, however does cause zinc concentrations to diminish.21 Prostatic secretions also contain a number of proteins. Their concentrations in the prostate are under androgen control and, again, serum concentrations increase with secretory volume until the age of 4 years, after which they gradually diminish.14 Chief amongst these proteins are acid phosphatase and canine prostate-specific esterase; again, the precise function of these enzymes remains unknown. Canine acid phosphatase is biochemically similar to that found in human prostatic fluid but is 100-fold less concentrated.25 Secretion is not confined to, and hence not specific for, the prostate, and acid phosphatase has been shown to also originate in greater concentrations from the epididymis in dogs.35 In contrast, canine prostate-specific esterase, a serum protease marker of secretory function,18 is virtually absent in human secretions but constitutes more than 90% of the total protein found in canine prostatic fluid. Canine prostate-specific esterase is reported to be closely related to the prostate-specific antigen protein.26,36 Acid phosphatase and canine prostate-specific esterase have proved to be useful markers of prostatic disease in men; however, although their secretion is very sensitive to androgen concentrations,39,52 it does not appear that there is any similarly useful correlation between variations in serum concentration and development of hyperplastic, inflammatory, or neoplastic prostate disease in dogs.7 The prostate gland begins to enlarge and function in the dog with the onset of puberty at 35 to 45 weeks of age.53 It continues to undergo progressive androgen-mediated enlargement throughout life as the consequence of benign prostatic hyperplastic changes; other than humans, the dog is the only species in which these changes consistently occur with increasing age. The normal prostate gland reaches its maximum normal cellular content by approximately 2 years of age. Benign prostatic hyperplastic is reported to develop beyond this age, with up to 16% of Beagles affected by that time; this incidence rises to 50% by 5 years and 70% by 8 to 9 years. Two histologically distinct forms of benign prostatic hyperplastic, glandular and complex, are recognized. Whereas the glandular form predominates in younger dogs (<4 to 5 years), complex hyperplasia is the more common form after that age.14 As the prostate increases in size, tissue dihydrotesterone concentrations decrease; this decrease matches the reduction in the declining secretory capacity of the gland from 4 years of age14,99 and the onset of complex hyperplasia. The decline in glandular dihydrotesterone content is also paralleled by an increase in the metabolism of androgens within the prostate gland and increasing numbers of nuclear androgen receptors; the prostate therefore becomes more responsive to androgens as it ages. Therefore, contrary to some suggestions,51 prostatic enlargement secondary to benign prostatic hyperplastic is not associated with higher tissue concentrations of dihydrotesterone, but there are increased numbers of receptors to dihydrotesterone. Significantly, mitotic figures are found with the same frequency in prostates affected by benign prostatic hyperplastic as in the normal gland, suggesting that its increased size is maintained not through an increase in cell proliferation but through a decrease in the rate of cell death. The changes of complex hyperplasia involve primarily the stromal elements and are characterized by asymmetric enlargement of the gland containing both glandular and prominent stromal elements. Within the stroma, areas of atrophy are common. The alveoli show cystic changes containing eosinophilic material; inflammatory cells, including lymphocytes and plasma cells, are evident. Ultrastructural changes include prominent microvilli, crowding with papillary projections, abundant granules, and caveolae in basal cells.37 Receptors for estrogen have been demonstrated in normal, hyperplastic, and neoplastic prostatic tissue.38,44 It is thought that estrogens also play a role in the pathogenesis of benign prostatic hyperplastic,29 although their role in the development of the prostate gland is not fully understood. In aging dogs, serum testosterone and prostatic dihydrotesterone concentrations diminish, but circulating estrogen concentrations remain unchanged; estrogens are therefore thought to increase the sensitivity of the prostate to dihydrotesterone by inducing nuclear dihydrotesterone receptors and promoting stromal and collagen synthesis.19 Administration of estradiol-17β with dihydrotesterone in young adult dogs to achieve the circulating concentrations found in aging dogs has been reported to induce complex hyperplasia with increased stromal elements.101 Estradiol and 5α-androstan-3α,17β-diol (3α-diol) have both been implicated in the development of benign prostatic hyperplastic in dogs and men and have been shown to amplify the effect of, or even substitute for, androgens.23 Estrogens may also exert an inhibitory effect in combination with dihydrotesterone on the rate of cell death, resulting in abnormal cell accumulation and other hyperplastic changes. In dogs with prostatic carcinoma, the number of estrogen receptors is significantly reduced, possibly accounting for a lack of differentiation of the tumor cells.44 History and Physical Examination A background history of the patient’s previous reproductive activity, including orchidectomy, the dog’s overall nutritional status, and any recent loss of condition investigated, should be reviewed. Significant presenting clinical signs include dyschezia, urethral bleeding, and pyrexia. Signs of orthopedic or neurologic abnormalities or discomfort on palpation of the hindlimbs and caudal abdomen should be investigated to determine if there is any relationship to prostatic disease. The penis should be examined for evidence of discharge (Figure 113-2) or trauma; acute urethral bleeding is encountered in patients with some prostatic diseases, but evidence of this may not always be present on physical examination. The testicles should be palpated for any evidence of change of size, consistency, or discomfort. Fluid samples for cytologic examination and microbiologic isolation may be collected from the prostate by ejaculate sampling, transurethral washing, or aspiration of fine-needle samples. Although ejaculate collection has been recommended in the investigation of prostatic infections,55 the relevance of the ejaculate sample itself is open to considerable debate because it is nonspecific for the prostate, containing in addition testicular and epididymal contributions, and may contain urethral contaminants. More importantly, the technique is often impractical in many dogs that are not familiar with or amenable to ejaculate collection and may cause considerable discomfort in patients with some inflammatory diseases. Transurethral prostatic massage samples may be useful in the diagnosis of generalized diseases of the prostate (e.g., inflammatory conditions) in which both the cellular and microbiologic content are likely to be representative. However, in cases of localized disease such as abscess, cyst, and neoplasia, their value is limited. In some cases, prostate fluid may not always be available,3 and transcatheter aspirate sampling has been advocated;5,55 however, the accuracy of this for localized lesions is not necessarily improved. Fine-needle aspirate samples may be collected percutaneously via a prepubic or, in a few cases, a perineal approach with the prostate gland fixed per rectum. However, this technique may lack the capacity for specificity in the case of some localized lesions. Fine-needle samples recovered under ultrasound guidance have therefore now taken precedence as the most widely used means of collecting samples for cytologic examination and microbiologic isolation. This technique allows the collection of samples for both investigations with minimal morbidity and a high index of specificity.74 Although there is some potential risk for “seeding” of neoplastic lesions along the needle tract,70 its minimal morbidity and accuracy for localized lesions, coupled with the high index of diagnostic specificity, argue strongly in its favor as the primary interventional investigative technique. Radiography can provide valuable information with regard to the size, position, and shape of the gland. The prostate glands of immature and castrated dogs are difficult to distinguish on plain lateral radiographs of the abdomen because of their small size and, in the former instance, their pelvic position. Prostates of adult dogs have conical ovoid silhouettes tapering at their caudal limit, which is found immediately cranial to the pubic brim. The gland is normally surrounded by periprostatic adipose tissue and hence appears to have a radiolucent margin that allows it to be distinguished from the colon dorsally, bladder cranially, and abdominal wall ventrally (Figure 113-3). Almost all prostatic diseases cause enlargement of the prostate gland that variably displaces the colon or bladder. Accumulation of feces in the colon may point to obstruction of colonic material. A lack of definition of the prostatic outline and other caudal abdominal organs may be seen with conditions that give rise to localized inflammation or peritonitis, including prostatitis and prostatic abscessation. Changes in the density of the prostate are sometimes recognized. Emphysematous changes may be seen associated with prostatitis.80 Mineralization within the prostate is likely to be associated with neoplastic disease33 in castrated dogs (Figure 113-4) but is less indicative in intact dogs.11 Small deposits are seen in prostatic calculi and mineralization in the capsule of discrete cysts located in “paraprostatic” positions may give rise to a characteristic appearance.79 Retrograde contrast studies (air or iodine) can amplify the information from plain studies and, in some inflammatory and septic conditions (e.g., prostatitis), allow the prostatic secretory systems to be highlighted, providing information with regard to the condition of the parenchyma itself (Figure 113-5). These studies also permit the bladder and prostatic urethra to be visualized and consequently the position of the prostate; conditions that result in its displacement (e.g., perineal region, cyst displacement) may therefore be usefully investigated with this technique (Figure 113-6). Figure 113-5 Prostatitis: pneumocystogram and urethrogram demonstrating parenchymal cavitation communicating with urethra. Ultrasound examination is capable of providing more combined information about the prostate gland than any other single imaging modality. It can be used to distinguish size, position, and margination and to provide information about the condition of the parenchyma and capsule itself, including symmetry, echodensity, and cavitation.34,48 Moreover, the real-time nature of this assessment increases the accuracy of other diagnostic interventions, including fine-needle and urethral wash sampling. The normal prostate gland of adult dogs has a homogenous appearance perforated by the central hypoechoic signal of the urethra. The separation between the two lobes is normally visible. In immature and castrated dogs, the parenchyma is more hypoechoic and the separation of the lobes less distinct (Figure 113-7). Figure 113-7 Sagittal ultrasound image of involuted prostate gland in an adult dog neutered as a puppy. Changes in BPH include an increase in the overall gland size, a heterogenous increase in echodensity, and small focal areas of echolucency (Figure 113-8). Cystic changes are common in patients with BPH and appear as multiple areas of very low echogenicity (Figure 113-9). In young dogs, the presence of fluid within the cystic areas may be associated with hematocystic lesions. Prostatitis may develop in the presence of multiple cystic changes; a combination of focal hyperechoic and hypoechoic areas is seen. Some areas are intensely hyperechoic and surround pockets of fluid accumulation (Figure 113-10). Abscessation appears as a progression of this with highly characteristic hyperechoic capsular and loculated tissue containing a fluid signal and hyperechoic flocculent material (Figure 113-11). Discrete prostatic cysts have a hyperechoic fluid ultrasound appearance48,91 that may be difficult to distinguish from that of the bladder (Figure 113-12). The cyst may be separated from the bladder by a hyperechoic pedicle or intimately involved with the parenchyma with discernible urethral communication. Neoplastic infiltration of the gland results in heterogenous hyperechogenic changes (Figure 113-13); concurrent cysts or abscessation may be present. Ultrasonography permits a number of interventional procedures for the prostate, most notably ultrasound-guided needle aspirate sampling. Aspirates can be performed under sedation or light anesthesia using 22-gauge spinal needles of lengths up to 2 to 3 inches and that contain a stylet to prevent sampling of unwanted tissues from the needle approach tract. The needle is guided in real time to the area(s) of interest and multiple aspirate samples recovered. Where prostatitis or abscessation is suspected, it may prove useful to use needles of a somewhat larger diameter (18 to 20 gauge) to permit aspiration of the purulent material; aspiration of discrete cysts can also be performed. Considerable care should be exercised, however, when aspirating abscesses and cysts so as to avoid lacerating the cyst or abscess wall and promoting postaspiration drainage into the peritoneal cavity; the availability of immediate surgical intervention is therefore wise. Therapeutic ultrasound-guided drainage of both cysts and abscesses has been described;10,15 replacement of the fluid or pus with alcohol or antibiotic solutions to further promote resolution of the lesion may be worthy of consideration. Similarly, aspiration of enlarged regional (medial iliac) lymph nodes can be undertaken during the same ultrasound procedure to confirm the presence of inflammatory change or to stage neoplastic disease. Figure 113-8 Benign prostatic hyperplasia. Sagittal ultrasound image showing homogenous prostatic enlargement. Figure 113-9 Cystic hyperplasia. Sagittal ultrasound showing heterogenous echodensity with small cystic areas. Figure 113-10 Prostatitis. Sagittal ultrasound image of prostate gland showing heterogenous changes through the gland.
Prostate
Anatomy
Embryology
Size
Anatomic Relations
Vessels
Nerves
Histology
Physiology
Hormonal Regulation of Prostatic Growth
Diagnostic Approach to Prostatic Disease
Microbiologic and Cytologic Samples
Diagnostic Imaging
Radiography
Ultrasonography
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Prostate
Only gold members can continue reading. Log In or Register to continue