Chapter 51 Probiotic Agents
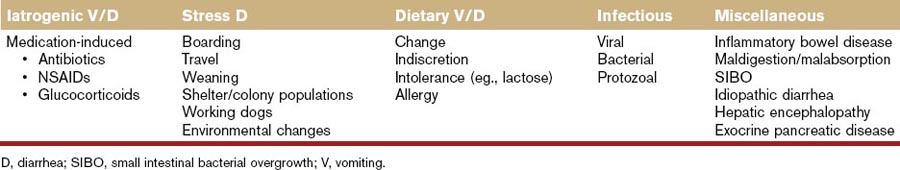
Probiotic agents are living organisms with low or no pathogenicity that exert beneficial effects on the health of the host. The use of probiotic agents (Box 51-1) has been advocated for general health as well as the prevention and treatment of many disorders in humans and in domestic and nondomestic animals.1 Although controlled studies documenting the efficacy of probiotics in dogs and cats are relatively lacking, the strongest and most consistent evidence of beneficial effects resulting from probiotic administration has been related to prevention and therapy of disorders of the digestive system (Table 51-1).2–4 Probiotics have been used as single-agent therapy or in conjunction with prebiotics as synbiotic therapy (Box 51-1).5,6
Table 51-1 Canine and Feline Gastrointestinal-Related Disorders Most Likely to Benefit from Probiotic Therapy
Probiotic Mechanisms of Action
Multiple mechanisms have been proposed to explain the beneficial effects of probiotic therapy, but most theorize an interaction between probiotic organisms and the indigenous gastrointestinal (GI) flora. Colonization of the intestine, particularly the colon, with bacteria from the environment occurs in the neonate during the first few days of life.2,7,8 Depending upon the specific organism and the host, these bacteria may preferentially and permanently establish residence in the lumen, mucous gel layer, crypts, or epithelial cell surface of the GI tract.9 Some bacteria provide benefit to the host through enhancement of immune responses and protection against potentially damaging agents.2 With few exceptions, probiotic agents establish only short-term residency in the GI tract following consumption by the host. Probiotic organisms bind to epithelial Toll-like receptors thereby triggering specific innate immune responses, depending upon the particular receptor, anatomic site, and host.10,11 Box 51-2 outlines other proposed mechanisms.12–14
Box 51-2
Probiotic Mechanisms of Action Related to the Gastrointestinal Tract
Stimulate innate protective immune responses of host GI tract
Upregulation or downregulation of GI immune responses through Toll-like receptors
Initiate production of antiinflammatory cytokines
Suppress production of proinflammatory cytokines
Alteration of GI epithelial cell function
Promotes repair of damaged epithelial cells
Aids in epithelial cell production of antibacterial substances and protective proteins
Inhibits epithelial cell apoptosis induced by cytokines
Competitive inhibition of GI attachment of pathogens and toxins
Prebiotic Effects on Probiotic Therapy
Fiber-containing prebiotic agents can augment probiotic therapy, although the simple addition of fiber to the diet or as a supplement does not automatically guarantee effective prebiotic activity as the effect varies with fiber type. Two classes of oligosaccharides, oligofructose (fructooligosaccharide) and inulin, are soluble fibers that are considered classic and effective prebiotic agents.15 Both classes of oligosaccharides are fermented in the colon. Oligofructose, which can be found naturally in soybeans, oats, beets, and tomatoes, undergoes rapid fermentation in the colon and provides the most benefit to bacteria residing in the proximal colon. Inulin, which is found naturally in plants such as Jerusalem artichoke, jicama, and chicory root, undergoes slower fermentation in the colon, and provides benefit to bacteria residing in both the proximal and distal colon. Although the dog and cat small intestines have minimal ability to digest prebiotic oligosaccharides, ileal and colonic bacteria are quite capable of fermenting these soluble fibers to short-chain fatty acids (acetate, butyrate, propionate) that provide metabolic fuel for colonic epithelial cells. Short-chain fatty acids decrease colonic pH resulting in pathogen inhibition, stimulation of sodium and water absorption, and suppression of abnormal colonocytes.16
Selection of a Probiotic Preparation
Labeling
When selecting a probiotic preparation for use in the dog or cat, a number of factors should be considered. One of these factors is the lack of regulation by the Food and Drug Administration (FDA)because probiotics are not presently classified as pharmaceutical products. Despite the advances of the past decade, most of the commercially available probiotic preparations, whether marketed for use in humans or animals, do not have adequate studies supporting manufacturer claims. Moreover, product labels have been shown in some instances to be inaccurate or incomplete in their identification and quantification of viable microorganisms.17 Consequently, it is advisable to start the selection of a probiotic agent by using a product marketed by a reputable, science-based company. By observing this principle, the preparation selected is more likely to satisfy basic criteria for safety and efficacy (Box 51-3).12,13,17,18 In addition to safety and efficacy data, the manufacturer’s label should have information on content, handling, and advisory statements (Box 51-4).12
Box 51-3
General Safety and Efficacy Criteria for Probiotic Agents in Commercial Preparations
Present in large numbers (>1 × 108 colony-forming units)
Resilient to technical processing
Able to survive gastric acidity and bile during GI transit
Capable of establishing temporary residence within the GI tract
Nonpathogenic, noncarcinogenic, and nontoxic
Incapable of absorption into the systemic circulation following consumption
Box 51-4
Important Probiotic Product Labeling Information
Product Contents
Number of live, colony-forming units per unit weight of product for each probiotic agent listed
Identification of fermentable fiber types if prebiotic agents included in product
Product Handling and Advisory Information
Accurate description of effect on host following consumption
Warning information regarding potential adverse drug or medical condition interaction with product
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


