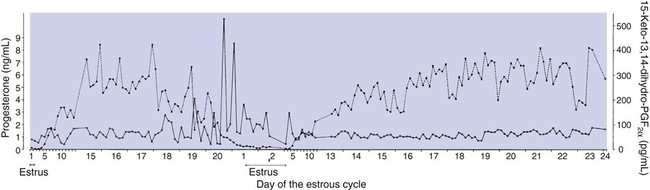
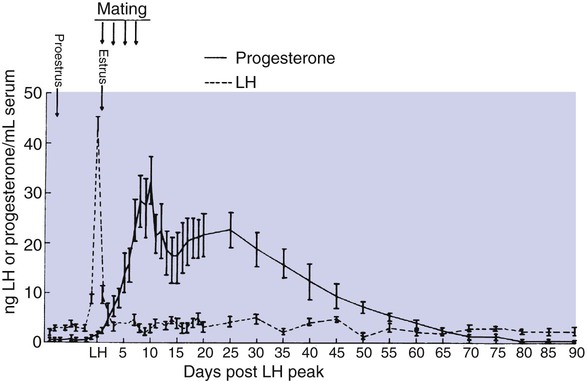
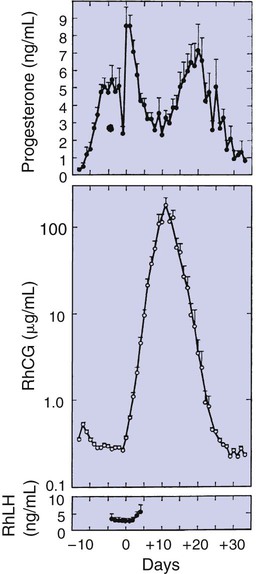
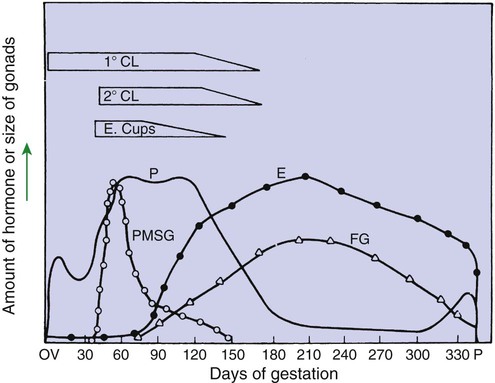
1. The development of an embryo involves fusion of an oocyte and spermatozoon within the oviduct. 2. Extension of the life span of the corpus luteum in large domestic species and cats is essential for pregnancy maintenance. 3. The placenta acts as an endocrine organ. 1. Fetal cortisol initiates delivery through increased secretion of estrogen and thus prostaglandin F2α. For those domestic animals (cattle, goats, horses, pigs, sheep) whose luteal activity is controlled by the uterus, modification of uterine prostaglandin F2α (PGF2α) synthesis and release is critical for the establishment of pregnancy. The embryo apparently produces substances that modify uterine production of PGF2α. Estrogen synthesis by the embryo is one way the endometrium may be informed regarding the presence of an embryo. A specific protein of embryonic origin called trophoblastin, produced before day 14 of pregnancy (or postovulation) in both sheep and cattle, is of immunological interest for the establishment of pregnancy; it has a close structural relationship to the molecule interferon. Movement of the embryo(s) in the tract is also important for pregnancy recognition. In the mare the embryo moves throughout both horns before being fixed at day 16. In pigs a minimal number of embryos need to be present (about four), presumably to occupy a sufficiently large area of the endometrium. Litter-bearing animals also use transuterine migration to maximize the opportunity for fetal development, a procedure that aids the recognition of pregnancy process. The end result is either suppression of PGF2α synthesis, as seen in the cow (Figure 38-1), or modification of the secretion mode (continuous instead of pulsatile), as seen in sheep. The absence of pulsatile secretion of PGF2α seems to be critical for the extension of the life span of the CL and the establishment of pregnancy in large domestic species. The dog does not extend its luteal phase during pregnancy; the luteal phase in the nonpregnant animal is often slightly longer (70 days) than in pregnant animals. Nevertheless, enhancement of luteal activity occurs through a placental luteotropin, likely relaxin, with progesterone secretion enhanced beginning at about day 20 of gestation or a few days after implantation. Early in the luteal phase, luteal function in the bitch is likely autonomous. During the second half of the luteal phase, luteinizing hormone (LH) and prolactin are likely luteotrophs (Figure 38-2). The rescue of the CL at the onset of pregnancy in primates involves the production of a luteotropin called chorionic gonadotropin (CG; for humans, hCG), which is produced by trophoblastic cells (syncytiotrophoblasts) of the embryo (Figure 38-3). For trophoblast tissue to produce CG, it must have intimate contact with the interstitium of the endometrium. This contact occurs by a type of implantation called interstitial, in which the embryo penetrates the endometrium at about 8 to 9 days after fertilization in humans and nonhuman primates. Secretion of CG begins 24 to 48 hours after implantation, with immediate enhancement of luteal progesterone production. Rescue of the CL in human pregnancy occurs as late as 4 to 5 days before the end of the luteal phase. The production of estrogen in the mare also involves an interaction between the placenta and fetus (Figure 38-4). From the work of Pashen and Allen, we know that the fetal gonads replace the fetal adrenals in primates as the key fetal endocrine organ involved in the cooperative synthesis of estrogen. The interstitial cells of the gonads appear to be the interactive cells, with fetal gonads enlarging to a size greater than the maternal gonads during the latter part of gestation. The production of estrogens during pregnancy in other domestic species, occurring relatively late in gestation, may involve the development of placental enzymes that allow progesterone to be metabolized to estrogens without the direct intervention of a fetal endocrine organ. (Fetal cortisol, however, is important for the induction of these placental enzymes, particularly in sheep; see next section.)
Pregnancy and Parturition
Pregnancy
The Development of an Embryo Involves Fusion of an Oocyte and Spermatozoon Within the Oviduct
Extension of the Life Span of the Corpus Luteum in Large Domestic Species and Cats Is Essential for Pregnancy Maintenance
The Placenta Acts as an Endocrine Organ
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pregnancy and Parturition
Only gold members can continue reading. Log In or Register to continue