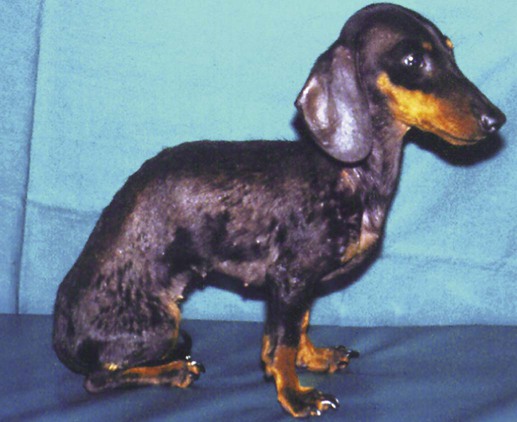
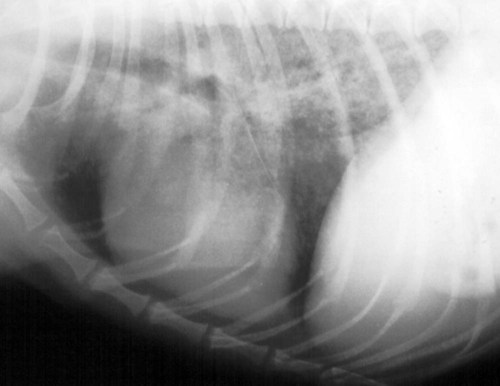
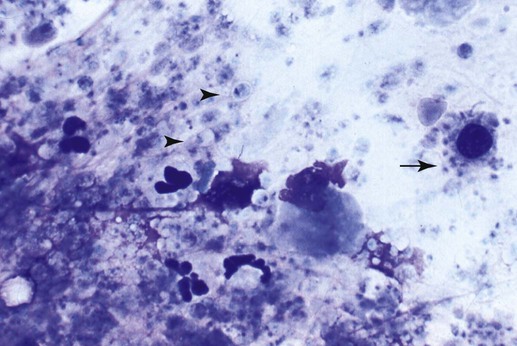
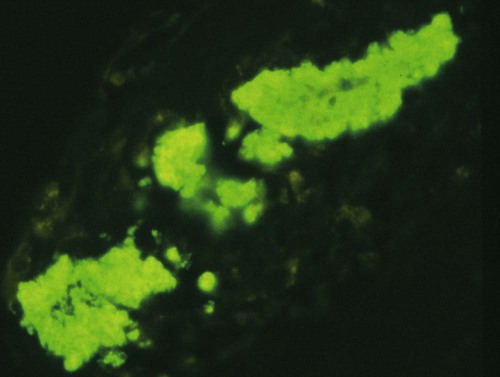
Pneumocystis carinii, the etiologic agent of pneumocystosis, occurs worldwide and infects virtually all mammalian species, including humans. Pneumocystis is a saprophyte of low virulence that exists in temperate and tropical climates at altitudes up to 5000 feet. Its primary habitat is the mammalian lung where it causes opportunistic pneumonia. Estimates of latent human infections range from 1% to 10% of the general population. Higher (greater than 50%) prevalence of subclinical infection is found using molecular studies on humans dying from other causes.49 Subclinical or latent infections are common in rats, mice, guinea pigs, rabbits, cats, sheep, and various wild animals. Clinical pneumonia has been reported to occur spontaneously in dogs, pigs, horses, goats, nonhuman primates, and humans. Airborne transmission is suspected as healthy animals become infected when they are housed with infected animals.5 The presumption is that the organism may have a yet-undiscovered dormant life stage in the environment. Most reports of clinical P. carinii pneumonia are linked with documented or suspected immunodeficiency syndromes in the host. The taxonomy of P. carinii is uncertain. It has been classified using freeze-fracture techniques as a unicellular protozoan belonging to the phylum Sarcomastigophora, subphylum Sarcodina. Ultrastructurally, the reproductive behavior of P. carinii is similar to the ascospore formation of yeast cells, and its organelles and staining properties by light microscopy resemble those of most pathogenic fungi. Phylogenetic classification based on 16S-like rRNA sequences indicates that P. carinii is most closely related to the fungi of the class Ascomycetes, especially Saccharomyces cerevisiae. Biologically, however, it behaves similar to a protozoan, because it is sensitive to drugs used to treat sporozoan infections but resistant to most antifungal drugs. The morphology of the organisms and the histopathology of the lesions produced by both human and animal isolates throughout the world are similar. Only a single species name has been assigned to the genus Pneumocystis, but antigenic differences suggest that several strains may exist. Although controversial, four species have been described: two species that infect dogs and rats, P. carinii and Pneumocystis wakefieldiae; one species that infects mice, Pneumocystis murina; and Pneumocystis jiroveci, which infects humans.32,56 The species found in the rat continues to be called P. carinii. Species designations for the organisms isolated from dogs and other animals will require further genetic and biologic study. Biologic differences between isolates from different hosts are suggested by the relative difficulty of experimental cross–host species transmission. For purposes of this chapter, and with few exceptions listed later in this text, the organism infecting dogs and humans will continue to be labeled P. carinii. P. carinii appears to be maintained in nature by transmission from infected to susceptible animals within a species. Although spore collections suggest an environmental source, none has been determined. The primary mode of spread is thought to be airborne droplet transmission between hosts. The contagious nature of pneumocystosis is suggested by the epidemic spread that has occurred in institutionalized humans. Sporadic case reports may represent an activation of latent infection by stress, crowding, and immunosuppressive therapy during hospitalization of latent carriers. Clinical disease has also been experimentally activated after glucocorticoid therapy, cytotoxic chemotherapy, and irradiation of laboratory rodents. A higher prevalence of infection has been found in dogs with canine distemper compared with a corresponding control population.57 Genetic analysis of isolates in humans indicates that most infections are not previously acquired but rather result from an infected source that is likely of the same species within a given geographic region.6 Therefore, recent acquisition of infection, rather than reactivation of infection, may be responsible for clinical illness.38 The greater prevalence of pneumocystosis is probably caused not only by an increased awareness of it, but also by the increased use of immunosuppressive therapy. The entire life cycle of P. carinii is completed within the alveolar spaces where organisms adhere in clusters to the lining cells. Ultrastructural studies have contributed a large body of information concerning the life cycle of P. carinii (Fig. 66-1). Two main forms, the trophozoite (1 to 4 µm) and cyst (8 µm), are found there. Although Pneumocystis infections are usually confined to the lungs, in human cases and an infection in one dog, organisms have been reported in extrapulmonary sites. Causes of severe cell-mediated immunodeficiency in humans, such as acquired immunodeficiency syndrome (AIDS), can be associated with lymphatic or hematogenous dissemination of organisms from the lungs to other tissues. Transmission of infection to an offspring can occur via aspiration of contaminated amniotic fluid from placental infection. Pneumocystis can be inhaled from the environment and then can colonize the lower respiratory tract of clinically healthy mammals; however, they rarely multiply to large numbers in the lungs of clinically healthy hosts. In conditions in which impaired host resistance (especially with reduced CD4 T-lymphocyte counts) or preexisting pulmonary disease is present, rapid proliferation of organisms can occur.34 The overgrowth and clustering of P. carinii within alveolar spaces can then lead to alveolocapillary blockage and decreased gaseous exchange. Intra-alveolar organims are often accompanied by thickening of alveolar septa, but they seldom invade the pulmonary interstitium and are rarely phagocytosed by alveolar macrophages. With an adequate immune response, the body may eliminate the infection, but the removal of large numbers of organisms and cellular debris may take up to 8 weeks. Both the presence of the organisms and the minimal inflammatory response they provoke contribute to the pulmonary alveolar damage. Extrapulmonary pneumocystosis has been reported in infected humans, but is extremely rare and occurs primarily when accompanied by overwhelming pulmonary infection, profound underlying immunodeficiency, and long-term use of aerosolized pentamidine for prophylaxis against P. carinii pneumonia in individuals infected with human immunodeficiency virus.62 Sites of extrapulmonary infection include lymph nodes, spleen, liver, bone marrow, gastrointestinal tract, eyes, thyroid gland, adrenal glands, kidneys, heart, pancreas, and external auditory canal. In general, pneumocystic infections of cats are latent or subclinical, as they are in humans. The organism has been found in the lungs of cats, but clinical disease has not been reported. Lung specimens examined from cats suffering from feline leukemia virus infection, interstitial pneumonia, or both have not shown evidence of pneumocystosis. Cats that were experimentally infected with P. carinii isolated from mice developed a cough, tachypnea, and pneumonia if they were immunosuppressed with concurrent glucocorticoid administration.63 In contrast, subclinical infections occurred in cats that did not receive immunosuppressive therapy. Most canine cases have been in miniature dachshunds younger than 1 year, with cases being reported in a Shetland sheepdog and Yorkshire terrier,11 and in adult Cavalier King Charles spaniels.9,12,50,58 Both the miniature dachshund and Cavalier King Charles spaniel have an underlying immune deficiency.39,61 Abnormalities on physical examination include dyspnea, tachycardia, and increased dry respiratory sounds on thoracic auscultation. Animals are usually in poor condition and cachectic, and they may show dermatologic changes, such as superficial bacterial pyoderma and demodicosis (Fig. 66-2). Although the mucous membranes are generally of normal color, they may be cyanotic in severely affected animals. Affected dogs remain relatively alert and afebrile, although slight (1° C to 2° C [1.8° F to 3.6° F]) elevations of rectal temperature have been reported. Fluid may be present in the thoracic and peritoneal cavities of some dogs. Ocular fundic lesions known as “cotton wool spots” rarely occur in humans with pneumocystosis, and they represent infarction of the nerve fiber layer. They have not been reported in animal infections. Hematologic abnormalities are usually nonspecific, and a neutrophilic leukocytosis with a shift suggesting inflammation is seen most consistently. Less frequently, eosinophilia and monocytosis are found. Polycythemia may occur secondarily to arterial hypoxemia from impaired gaseous exchange. Thrombocytopenia, which can be significant enough to cause bleeding, has been a complication in humans. In contrast, thrombocytosis may be present in miniature dachshunds. Artifactual thrombocytopenia and megathrombocytosis have been observed in Cavalier King Charles spaniels,27 which may have a hereditary basis likely unrelated to the suspected immunodeficiency. Biochemical alterations are usually nonspecific. Total serum proteins are usually within reference limits with a low to borderline-low globulin level, which correlates with low γ-globulin levels on serum protein electrophoresis.41 Arterial hypoxemia (oxygen tension [pO2] at or below 80), hypocapnia (carbon dioxide tension [pCO2] at or below 35), and increased arterial blood pH indicate an uncompensated respiratory alkalosis. The pO2 is often lower than would be expected from the clinical signs and thoracic radiographs. Findings on thoracic radiography include diffuse, bilaterally symmetric, miliary-interstitial to alveolar lung disease (Fig. 66-3), with compensatory emphysema in severely infected animals. Solitary lesions, unilateral involvement, cavitary lesions, spontaneous pneumothorax, and lobar infiltrates are occasionally present. Tracheal elevation, right-sided heart enlargement, and pulmonary arterial enlargement reflect cor pulmonale secondary to diffuse pulmonary disease. Although serologic tests are available for detecting human infections, their diagnostic value is uncertain, because many immunodeficient patients who develop pneumocystosis fail to produce antibody titers, and healthy individuals frequently have high titers. Increased antibody titers to P. carinii persist for long periods, offering a valuable index of infection in epidemiologic studies. However, they are of limited use for an immediate diagnosis.18 An increase in titer over 2 to 3 weeks is needed to confirm active infection. Circulating Pneumocystis antigen has been detected in human serum by counterimmunoelectrophoresis and enzyme-linked immunosorbent assay methods. However, antigenemia also is found in up to 15% of clinically normal humans who have been tested. P. carinii has been successfully propagated in cell cultures, but not on a continual basis. Immunosuppressed rodents have been used to propagate organisms for serologic and experimental testing. Because of the difficulty of isolation of organisms, diagnosis requires direct demonstration of P. carinii in biopsy specimens, respiratory fluids, or occasional extrapulmonary sites. Sputum, transtracheal or endotracheal washings, gastric contents, and oropharyngeal secretions may contain organisms. Transtracheal aspirates have been effective for identifying organisms in dogs.41 Other techniques for collecting cytologic samples are endobronchial brushing and transbronchoscopic biopsy; however, these procedures require special endoscopic equipment and involve the risks of general anesthesia. Transtracheal or endotracheal lavage and percutaneous transthoracic needle aspiration are more available to practitioners and have been shown to have good correlation with transbronchoscopic biopsy findings in confirming a diagnosis. None of the cytologic techniques are as reliable or as definitive as is histopathologic examination of lung biopsy specimens for documenting active pneumocystosis. Lung biopsy is unfortunately more invasive, has potential complications of hemorrhage or pneumothorax, and is associated with additional costs and hospitalization. Of 24 infected dogs reported in the literature, diagnosis using multiple procedures was made antemortem in 15 dogs. In one study, tracheal washing was performed in 9 dogs, and the organism was found in 7.35,41 Bronchoalveolar lavage results were positive in 1 of the 2 cases in which it was used.50,58 Transthoracic needle aspiration was performed in 3 dogs; 2 developed pneumothorax, and 1 died as a direct result of the procedure.23,50 The aspirate was diagnostic in only 1 of the dogs. Unfortunately, endoscopic or percutaneous lung biopsy has the greatest risk of complications. Hemorrhage, secondary infection, pneumothorax, and death from anesthesia have been reported after biopsy in dogs. Open surgical biopsy is the preferred method and gives a definitive diagnosis.23,27 The least traumatic and invasive method involves a laparotomy with a transdiaphragmatic approach.27 Antimicrobial therapy can begin 24 to 48 hours before specimen collection in a patient suspected of having pneumocystosis, without the drug masking the presence of organisms in the sample. To facilitate early diagnosis and treatment, impression smears for cytologic study should be made from all tissues before fixation for histologic evaluation. The cytologic material obtained is placed on a glass microscope slide to dry, after which it is selectively stained with methenamine silver for cysts or with Giemsa for nuclei of intracystic sporozoites and trophozoites (Fig. 66-4). Diff Quik (a modified Giemsa stain) can be used as a fast and inexpensive screening stain, after which negative results can be confirmed with more sensitive staining.16 Unfortunately, Pneumocystis organisms are difficult to detect in respiratory secretions or washings, and success in finding them usually depends on the experience of the examiner and the collection and processing of specimens. Direct or indirect fluorescent antibody test has been effective in specifically detecting organisms in sputum, tracheal aspirates, or pulmonary tissue (Fig. 66-5).47 Immunoperoxidase techniques also can be used to identify P. carinii in impression smears and in formalin-fixed, paraffin-embedded lung sections (Fig. 66-6, A and B).37 Polymerase chain reaction has been effective in the detection of P. carinii in bronchoalveolar lavage specimens from humans36,38,42,51 and in lung tissue from dogs.28,58 Sensitivity of polymerase chain reaction on bronchoalveolar lavage fluids from infected humans has been greater than that of conventional staining without loss of specificity.24 DNA analysis of Pneumocystis isolates has been used for epidemiologic monitoring of isolates from humans and animals.22
Pneumocystosis
Etiology
Epidemiology
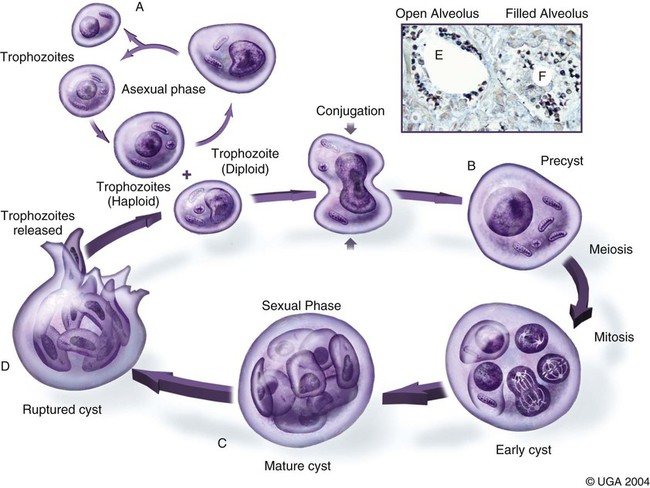
Pathogenesis
Clinical Findings
Cats
Dogs
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine