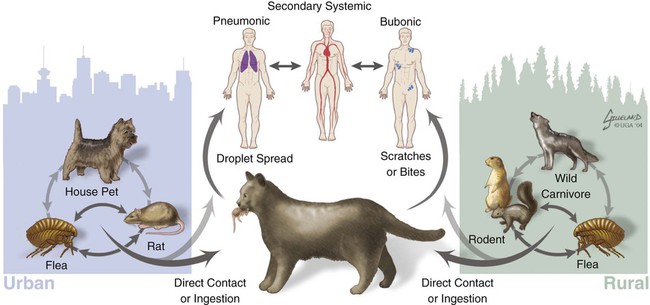
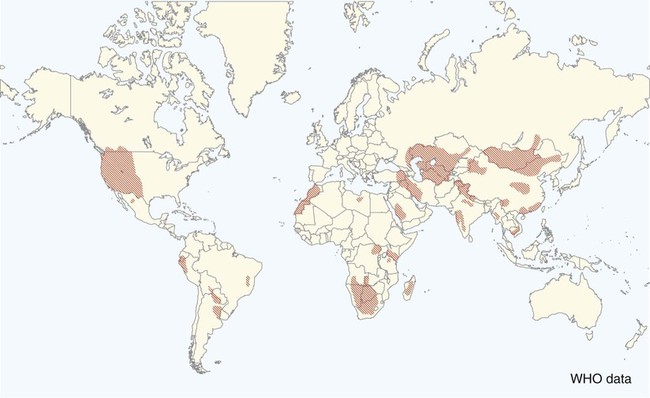
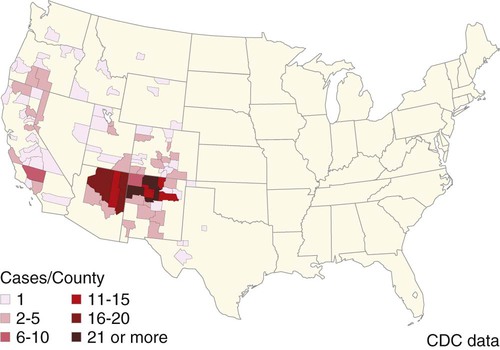
Plague is caused by Yersinia pestis, a coccobacillus of the family Enterobacteriaceae. It is a nonmotile, non–spore-forming, facultative anaerobic, gram-negative, bipolar-staining bacterium. The bacterium is nonsaprophytic and sensitive to desiccation and temperatures higher than 40° C. However, it can survive for several weeks to months in organic material such as infected carcasses, and survival in contaminated soil has been confirmed in natural conditions for at least 24 days and in experimental condition for up to 40 weeks.2,17 Cold temperatures or freezing may prolong the viability of this organism for years. The organism grows slowly in culture even at its optimum temperature (28° C on blood agar or MacConkey agar), so cultures should be held for at least 48 hours before being discarded. Colonies are much smaller than other Enterobacteriaceae and may be overlooked. As recently as 1500 to 20,000 years ago, Y. pestis evolved from Yersinia pseudotuberculosis, a relatively benign enteric bacterium that is transmitted through contact with contaminated food and water.16 As part of its transition from an enteric to vector-borne pathogen, Y. pestis has acquired two unique plasmids (pPla and pMT1), shed genes required for persistence outside the vertebrate or vector, and gained apparently new functions for several chromosomal genes. Y. pestis produces a lipopolysaccharide endotoxin and a capsular envelope containing the antiphagocytic principal fraction I antigen. Three geographic variants of Y. pestis (orientalis, antiqua, and mediaevalis) have been distinguished biochemically but have identical virulence. Plague, an infection of rodents, can be highly lethal and causes widespread epizootics. Although most commonly associated with rodents, nearly all mammals can become infected with Y. pestis. Human infection usually occurs when epizootics are affecting rodent populations or during accidental infection in enzootic areas. Infection is maintained by an obligatory flea–rodent–flea cycle involving bacteremic rodent hosts and their resident fleas (Fig. 45-1). More than 80 flea species have been found to be naturally infected with Y. pestis, and at least 28 flea species occurring in North America have been experimentally confirmed as vectors.14 The apparent diversity of potential mammalian hosts and flea vectors is misleading, however, as many species of mammals fail to develop the high bacteremia required to infect feeding fleas or harbor few potential flea vectors. Natural reservoir hosts are relatively resistant to plague infections, but susceptibility varies tremendously within a species and is based on geographic location, flea species, and environmental factors. In North American carnivores, seroprevalence is highest in mustelids; intermediate in ursids, felids, and canids; and lowest in procyonids.37a Cat and dog fleas (Ctenocephalides spp.) are considered poor vectors for plague. In the United States, prairie dogs (Cynomys spp.), rock squirrels (Spermophilus variegatus), and ground squirrels, such as Spermophilus richardsonii or Spermophilus beecheyi are commonly infected wild hosts and fleas such as Oropsylla montana are very efficient vectors of plague. Mortality in these rodent species approaches 100%. Plague exists in various discontinuous regions in Asia, Africa, and the Americas (Fig. 45-2). These foci of plague are most frequently associated with semiarid, cooler climates that are usually adjacent to deserts. Each focus is unique in terms of its rodent reservoirs, flea vectors, and climatic and environmental factors.34 Common characteristics of endemic areas include the availability of rodent hosts with a short life expectancy, high reproductive potential, and year-round flea activity. In the United States, plague foci are located throughout an area bounded on the east by the Rocky Mountains, with some incursions in the great Midwest plains, and on the west by the Pacific Ocean (Fig. 45-3). In reservoir rodent populations, the disease may have explosive and often devastating sporadic or periodic epizootics. Humans and pets exposed to infected rodents during these epizootics may develop plague. Eisen et al. created logistic regression models to identify landscape features associated with areas where humans have acquired plague from 1957 to 2004 in the four-corners region of the United States (Arizona, Colorado, New Mexico, and Utah), and extrapolated those models within a geographic information system to predict where plague cases are likely to occur within the southwestern United States disease focus.15 The probability of an area being classified as high-risk plague habitat increased with elevation up to approximately 2300 meters (7546 feet) and declined as elevation increased thereafter, and declined with distance from key habitat types (e.g., southern Rocky Mountain piñon-juniper, Colorado plateau piñon-juniper woodland, Rocky Mountain ponderosa pine, and southern Rocky Mountain juniper woodland and savanna). Interannual variability of human plague incidence in these regions can be influenced by annual precipitation.1a Summer soil moisture favors flea survival and vegetation growth for rodent populations. Global climate warming will likely influence the distribution of plague, with an expected shift of endemic regions away from the equator.31b Transmission of Y. pestis between most hosts occurs by flea bite. Although less common, transmission can also occur through contact of the organism with mucous membranes or broken skin or by inhalation of droplets from animals with pneumonic plague (see Fig. 45-1). In contrast, cats and dogs usually acquire plague by ingestion of infected rodents or lagomorphs and less commonly by bites from their prey’s plague-infected fleas. As reported by Watson et al.,40 in examining formalin-fixed paraffin-embedded archival tissues of seven adult cats that died after being experimentally infected with Y. pestis, the lesions of the orally infected cats were consistent with those previously described for naturally occurring Y. pestis infections in cats. It corroborates the contention that cats most commonly contract plague by eating Y. pestis–infected rodents and not via flea bite. The histopathology of Y. pestis disease in these cats was comparable to that described for human plague.40 Of 60 cats diagnosed with plague, 75% had hunted rodents.13 Cats are highly susceptible to plague infection and usually develop bubonic plague, just like humans. However, septicemic and pulmonic plague can also occur in cats, the latter form being epidemiologically the most dangerous for humans.12,18 Twenty-three cases of cat-associated human plague (five of which were fatal) occurred in eight western states from 1977 through 1998, which represent 7.7% of the total 297 cases reported in that period.18 Bites, scratches, or other contact with infectious materials while handling infected cats resulted in 17 cases of bubonic plague, 1 case of primary septicemic plague, and 5 cases of primary pneumonic plague. The 5 fatal cases were associated with misdiagnosis or delays in seeking treatment, which resulted in overwhelming infection and various manifestations of the systemic inflammatory response syndrome. Ten days after ingestion of rodents infected with Y. pestis, plague bacteria can be isolated from the oropharynx and bloodstream in approximately 50% and 20% of dogs, respectively. Dogs develop only mild clinical signs, including fever and lymphadenomegaly, and undergo seroconversion. Contact with domestic pets and wild animals has been identified as a potential risk factor for human plague infection (see Public Health Considerations). A flea must first ingest blood from a highly bacteremic host (usually a rodent). Within days, the plague bacilli multiply within the flea’s proventriculus (a globular structure lying between the flea’s esophagus and stomach) and midgut, eventually forming a blockage in the proventriculus. The interior of the proventriculus is lined with a series of spines that mechanically lyses ingested erythrocytes, allowing smoother passage into the stomach and preventing ingested blood cells from flowing back toward the mouth of the flea. In some species of fleas, such as the Oriental rat flea, Xenopsylla cheopis, the proventriculus also serves as a site for initial colonization and eventual blockage (occlusion) of the flea gut by multiplying Y. pestis.23 Following ingestion of blood from an infected reservoir host, Y. pestis may be cleared by some fleas. In others, it replicates in high numbers in the flea stomach. The proventricular block formation is regulated by a group of chromosomal genes called the hemin storage locus (hms), which is upregulated at ambient temperatures and not expressed at 37° C. The hms is required for successful colonization of the proventriculus, but not for colonization of the midgut.16 In contrast, the Yersinia murine toxin (ymt), located on the newly acquired pMT1 plasmid, is a phospholipase required for survival in the midgut of the flea. Ymt activity arises within Y. pestis after exposure to plasma ingested with the blood meal. After several days of successful replication, bacterial cells aggregate and attach themselves to the lining of the proventriculus. In addition to proventricular blockage, biofilm can cause a partial blockage or may be useful for forming large bacterial aggregations that help prevent the bacteria from being passed in feces. Blocked fleas become starved for blood and attempt to feed more often. During these attempts, they regurgitate plague organisms into the bite wound of an uninfected host. Mainly blocked fleas transmit infection; they may die of starvation and dehydration. Fleas may remain infected for more than a year, allowing transmission of the disease long after the death of the host. The role of blocked fleas in epidemic or epizootic spread depends on the flea species involved, infestation rates, and host-seeking flea abundance.16 X. cheopis blocks quickly compared to most fleas and has been the standard model, but probably represents an exception, rather than the norm. A combination of early-phase transmission and blocking probably helps to explain why this flea is so dangerous, but other fleas that are important in epizootic spread probably rely mainly on early-phase transmission. In an experimental model to simulate oral exposure to plague in cats, healthy cats (n = 16) fed a mouse that had died of experimentally induced Y. pestis infection to simulate oral exposure to plague, either died (6 of 16 or 38%), developed transient illness and recovered (7 of 16 or 44%), or showed no signs of illness (3 of 16 or 19%).21 A continual fever (40.7° C to 41.2° C [105° F to 106° F]) was associated with a poor prognosis. The highest antibody titers developed in the group that shed the plague bacillus over an extended period of time. Blood, throat, and oral cavity cultures were positive in 100% of the fatal cases. Throat cultures were positive in 75% of the exposed cats. Most cats fed infected mice had Y. pestis cultured from their oropharynx and could have transmitted plague. In the natural environment, the mortality in cats approaches 50%. Cats with previous antibody titers to plague generally have a more protracted illness but are not protected from bacteremia or death. In summary, plague should be considered when examining any febrile cat in endemic areas. Other atypical clinical signs have included ocular discharge, vomiting, diarrhea, dehydration, weight loss, a poor haircoat, a swollen tongue, tonsillar enlargement, necrotic stomatitis, facial ulceration, cellulitis, and abdominal distention.7,13 Clinical illness is infrequently reported in dogs. Fever, anorexia, cervical and mandibular lymphadenomegaly and abscessation as well as coughing have been observed in naturally infected dogs.33 In a report of plague in 3 dogs, clinical signs included lethargy (3 dogs), pyrexia (2 dogs), and a purulent skin lesion in the cervical region (2 dogs).32
Plague
Etiology
Epidemiology
Pathogenesis
Clinical Findings
Cats
Dogs
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine