Isotope source of signal
Main γ energy (keV)
Period (h)
Labeled agent category
Target category
99mTc
140
6
• Antibodies
• Cells
• Ligands
• Nucleic acids
• Peptides
• Proteins
• Nanoparticles compounds
• Antigens
• Adhesion molecules
• Cells
• Enzymes
• Lipids
• Receptors
• Transporters
• Nucleic acids
123I
159
13.2
111In
171 and 245
67.2
201Tl
71
72.9
125I
27 and 30
1,437
Table 2
Examples of SPECT neurotracers with their radiopharmaceuticals acronyms, corresponding isotopes, targeted metabolisms and functions, commercial or laboratory availability and human or animal applications
Tracer name or acronym | Isotope | Metabolism and function [target] | Commercial, Laboratory (human, animal) | References |
|---|---|---|---|---|
SESTAMIBI | 99mTc | Tumor (glioma) [cellular viability: P-glycoprotein, MDR-1] | c (h) | (27) |
TETROFOSMIN | 99mTc | Tumor (glioma) [cellular viability: cytosol and mitochondria] | c (h) | (30) |
THALLIUM | 201Tl | Tumor (glioma) [cellular viability: Na/K-ATPase pump and membrane potential] | c (h) | |
DTPA-Bis | 99mTc | Tumor (glioma) [methionine] | Laboratory (a) | (35) |
CXCR4 | 125I | Tumor (glioma) [chemokine receptors] | Laboratory (a) | (33) |
RGD | 99mTc | Tumor angiogenesis (glioma) [αvβ3 integrin expression] | Laboratory (a) | (32) |
IMT | 123I | Tumor (glioma) [tyrosine] | Laboratory (h) | (29) |
HMPAO | 99mTc | Brain perfusion [astrocytes binding] | c (h, a) | (11) |
ADAM | 123I | Neurological and psychiatric disorders [serotonin transporters] | c (h, a) | |
I-CO | 123I | Neurological and psychiatric disorders [5-HT2a serotonin receptor] | Laboratory (h, a) | (19) |
BOMBESIN | 99mTc | Neurotransmitter Tumor Transplanted cells [bN receptors] | Laboratory (a) | |
ANNEXIN-V | 99mTc | Neurodegenerative disorders Apoptosis-cell stress Ischemia-stroke [phosphatidylserine] | c (h, a) | (21) |
IMPY | 123I | Alzheimer disease [β amyloid plaques: thioflavine-T-derivative] | Laboratory (a) | |
IBZM | 123I | Neuronal dopaminergic system [D2 dopamine receptors] | c (h, a) | |
FP-CIT | 123I | Neuronal dopaminergic system [presynaptic dopamine transporters] | c (h, a) | |
IOMAZENIL | 123I | Neuronal activity [benzodiazepine receptors] | c (h, a) | (7) |
2 Materials
2.1 SPECT Imaging Instrumentation
SPECT images were acquired using the eXplore speCZT Vision 120 preclinical imaging system (GE Healthcare, Waukesha, USA) illustrated in Fig. 1. The SPECT part consists in a full ring of semiconductors, CZT detectors (10 heads of 8 cm × 8 cm, pixel size 2.46 mm, energy range 20–200 keV, and energy resolution <7% at 140 keV), and rotating multi-pinhole dedicated tungsten collimators for mouse or rat. For mouse imaging, radial field of view (FOV) is 34 mm with a cylindrical multi-pinhole collimator of seven pinholes of 1 mm diameter each (Note 3). For rat imaging, radial FOV is 78 mm using a cylindrical multi-pinhole collimator of five pinholes of 1.5 mm diameter each. Using the helical acquisition mode, the axial FOV can be adjusted to the size of the head and neck of the small rodent but at the expend of longer acquisition times.
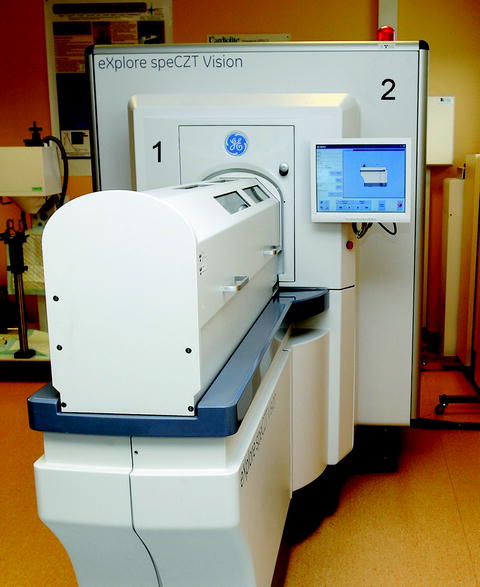

Fig. 1.
View of the combined SPECT/CT preclinical imaging system used (eXplore SPECZT vision 120) with the SPECT part (1) in front and CT part (2) in the back. Functional and anatomical images are acquired sequentially after translation on the same axis of the imaging cell from one modality to the other.
2.2 Anesthesia, Imaging Cell and Animal Monitoring Instrumentation
Small animals (mice and rats) are anesthetized with a gas mixture (air and 1–2% isoflurane) in an air warmed-up multimodality imaging chamber (Minerve, Esternay, France) to keep constant at 37°C their temperature. Heart rate is obtained through three paws carbon tube electrodes (Note 4) fitted with conductive gel and insensitive to both X-rays or magnetic field as previously described (39) and connected with an electrocardiograph (ODAM Physiogard RSM 784, Wissembourg, France) delivering a physiological trigger corresponding to the QRS of the ECG signal. Monitoring of breathing rate is obtained via a small differential pressure transducer (TSD 110-MRI; Biopac Systems Inc., CA, USA) placed under the thorax of the animal lying in prone position (Note 5).
2.3 Multimodality Instrumentation for Image Co-registration (SPECT/CT and SPECT/MRI)
Micro-SPECT functional images can be fused with anatomic images issued from other imaging techniques as micro-CT (Note 6) or micro-MRI (Note 7). The image fusion allows a correct anatomic location of the radioactivity uptake, which is of particular importance in brain imaging. Translation of the animal from one modality to the other is achieved using the imaging cell and without moving the animal, avoiding then the use of fiducial markers. A close proximity of both imaging systems or better a co-axially dual modality device configuration is therefore recommended.
The CT part of the eXplore speCZT Vision 120 illustrated in Fig. 1 and which we used for multimodal imaging is placed on the same axis and back-to-back with the SPECT and has a high-power rotating anode X-ray tube (focal spot of 50 μ, 120 kV maximum potential, and 50 mA maximum current). X-rays attenuation projections are obtained with a large area CCD detector bonded to a CsI-coated fiber optic taper.
When high-field MRI is considered for morphological co-registration with SPECT, there could be complex physical interactions between the high magnetic field generally used and the nuclear instrumentation preventing then their close proximity (Note 8). For that reason, we developed a low-field MR imager dedicated to preclinical multimodality imaging, Fig. 2 (40). The main magnetic field B 0 of 0.1 T is oriented vertically and delivered by a small water-cooled open electromagnet (Bouhnik SAS, Velizy, France). The field homogeneity ΔB/B 0 = 5 × 10−6 is obtained in an imaging volume of 10 × 10 × 6 cm3 with maximum encoding gradients’ strength of 20 mT/m that are reached in 0.5 ms. Radio frequency (RF) transmit-and-receive solenoid coils are made by adjacent loops of copper wire tuned at 4.26 MHz corresponding to the Larmor frequency of the 1H at that field.
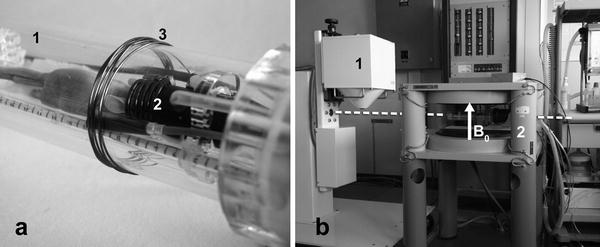

Fig. 2.
(a) Close-up view of the nonmagnetic technical cell dedicated to small animal imaging adapted for multimodality showing a mouse inside the cell (1), the RF coil around his head (2), and the pick-up coil (3) around the imaging cell for transmission and reception of the RF pulses to the solenoid coil. (b) SPECT/low-field MRI dual imaging system with the pinhole SPECT camera (1) adjacent on the same axis (dashed line) to the magnet of the low-field MRI system (2), orientation of the main magnetic field B 0 is indicated by the arrow. Functional and anatomical images are acquired sequentially after translation of the imaging cell from one modality to the other.
RF pulses are transmitted to the solenoid coil by a pick-up copper coil rounded outside the imaging cell (as illustrated in Fig. 2), which also receive the encoded MR signal. For anatomical images, co-registration a T1-weighted 3-dimensional (3D) sequence was developed on a SMIS (Guilford, UK) console to achieve typical isotropic (Note 9) spatial resolutions of, respectively, 1 × 1 × 1 mm3 for rat and 0.5 × 0.5 × 0.5 mm3 for mouse. The low-field MR imaging system was used back to back with a small animal SPECT gamma camera (Gaede Medizinsyteme, Freiburg, Germany) equipped with one pinhole of 1.5 mm in diameter and a focal distance of 12 cm (41).
2.4 SPECT Images Reconstruction Software
The Microview software driving the eXplore speCZT Vision 120 permitted the SPECT/CT images reconstruction. A 3D OSEM (11 averages and 5 subsets) algorithm, without Compton and attenuation correction, was applied for micro-SPECT images reconstruction leading to reconstructed voxels of 300 × 300 × 300 μ3. A cone beam filtered back-projection Feldkamp algorithm is used for micro-CT images reconstruction leading to isotropic reconstructed voxels of 100 × 100 × 100 μ3. For the SPECT/low-field MRI experiment, the SPECT images were reconstructed using an algebraic reconstruction technique (ART) enabling an isotropic 1 × 1 × 1 mm3 spatial resolution and a voxel size of 470 × 470 × 470 μ3 (42).
2.5 Hot Lab Equipment for Radiopharmaceutical Preparation and Quality Control
Hot lab equipment needs to meet each national nuclear regulatory standards for single photon emitters biological applications including:
Shielded cabinet(s)
Dose calibrator for accurate radionuclide activity measurements
Shielded syringes
Radiochromatograph and chromatography strips for radiochemical QC
Shielded waste container
Low energy shielded decay drums
Small animal QC SPECT phantoms
Contamination monitor and personal dosemeter monitor(s)
Radioactive waste management systems (for collection of radioactive liquid and organic waste)
3 Methods
3.1 Radiopharmaceutical Preparation Steps
Detailed data on numerous SPECT radiopharmaceuticals chemistry and labeling preparation steps can be found in the MICAD database (Note 10). In the following, as typical examples, we will only focus on the principal preparation and quality control steps of two commercial radiopharmaceuticals (respectively, 99mTc-HMPAO and 123I-FP-CIT) that will be illustrated in small animal SPECT for imaging brain perfusion and dopamine transporters.
3.1.1 99mTc-HMPAO: A Brain Perfusion Agent
The lipophilic complex 99mTc-Exametazime or formerly 99mTc-Hexamethylpropylene amine oxime (HMPAO) (Ceretec®, GE Healthcare, Little Chalfont, UK) is a pre-dispensed sterile, nonpyrogenic, lyophilized mixture of 0.5 mg [(RR-SS)-4.8-diaza-3, 6,6,9-tetramethilundecane-2,10-dione bisoxime] which is reconstituted with a solution of 99mTc sodium pertechnetate. Sterile technique must be used throughout.
1.
Place the vial in a suitable shielding container and swab the rubber septum with the sterile swab provided or with an appropriate disinfectant (e.g., chlorexidine, benzalkonium chloride).
2.
Add 0.37 GBq up to 2 GBq of 99mTc to the vial using an eluate from a 99mTc generator which was eluted within 30 min. Use only elute from a technetium generator which has been eluted within 24 h. Before reconstitution, the technetium generator elute may be adjusted to the adequate desired specific activity in a volume of 5 mL by dilution with sodium chloride solution USP for injection.
3.
Inject 5 mL of sterile elute into the shielded vial using a 5 mL syringe. Before withdrawing the syringe from the vial, withdraw 5 mL of gas from the space above the solution to normalize the pressure in the vial. Gently invert the shielded vial for 10 s to ensure complete dissolution of the powder.
The useful life of the reconstituted agent is limited to 30 min.
Quality controls must be performed according to the following points:
Preparation must be an aqueous, clear, and colorless solution.
pH of the prepared injection should be in the range 9–9.8.
Radiochemical purity greater than 80% is mandatory for product acceptance.
Control of radiochemical purity is performed using chromatography strips (model 150-130, Biodex Medical Systems Inc.) with ethyl acetate as solvent. First, prepare a chromatography tube containing ethyl acetate to saturate it. Then apply at least 5 μL samples of Ceretec® to the origin of the strip within 15 min of reconstitution. Immediately place one strip into the ethyl acetate tube and make sure that the strip is not adhering to the side of the test tube. When elute has reached the solvent front, remove the strip from the tube and count the strip with a suitable radiochromatograph equipment (Minigita, Raytest Isotopenmessgeräte GmBH, Straubenhardt, Germany).
3.1.2 123I-FP-CIT: A Dopamine Transporter Imaging Probe
Ioflupane or [123I]-N–ω-fluoropropyl-2β-carbome-thoxy-3β-(4-iodophenyl)nortropane (Datscan®, GE Healthcare, Little Chalfont, UK) is an injectable “ready to use, 74 MBq/mL” specialty. Vial of 2.5 mL have 4.5 × 1014 specific activity. Before being placed in the shielded enclosure, vial is disinfected with 2-propanol. The septum is disinfected with an appropriate disinfectant (e.g., chlorexidine, benzalkonium chloride) immediately before to take a sample for IV injection. Check for radiochemical purity as detailed above.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


