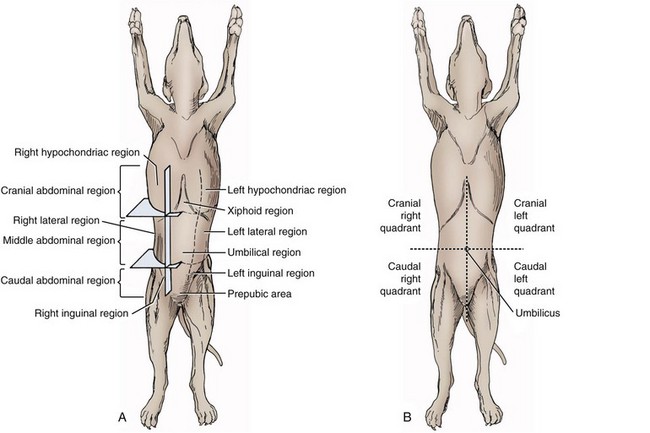
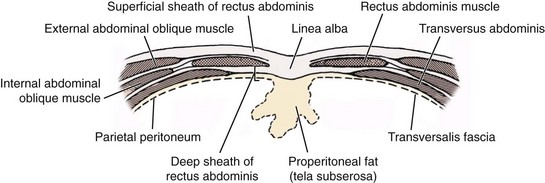
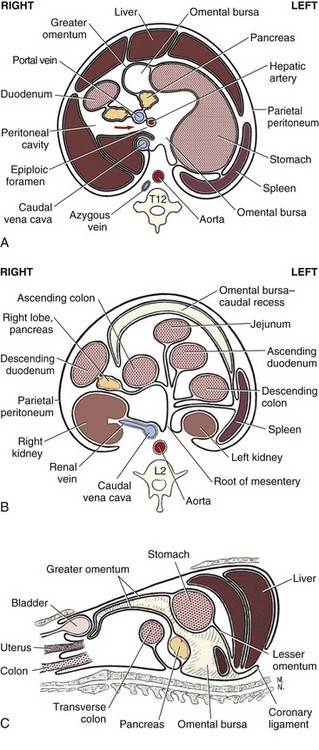
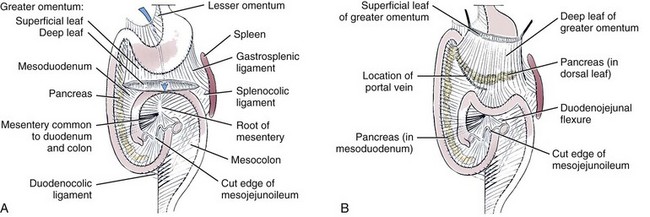
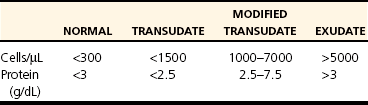
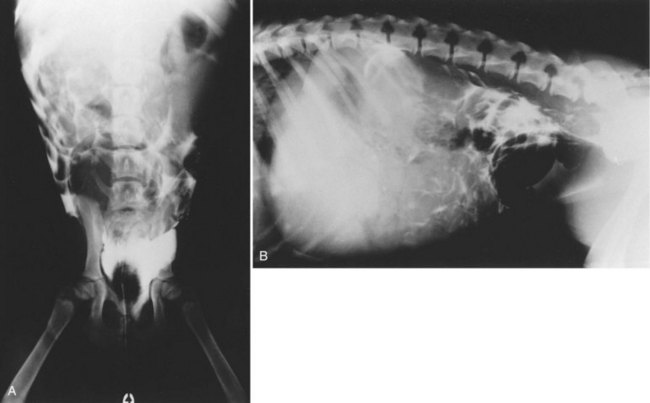
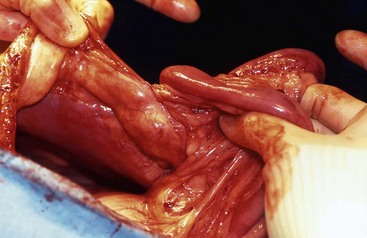
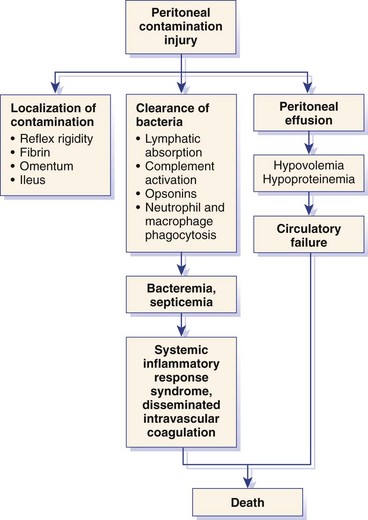
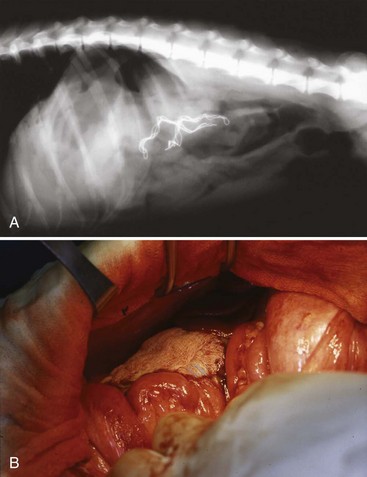
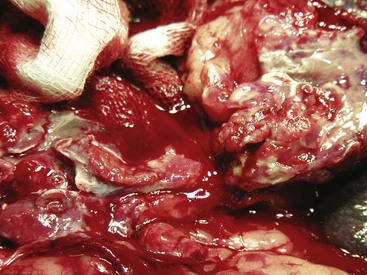
Chapter 86 The peritoneal cavity develops in the embryo from the lateral mesoderm.235 Two sheets of mesoderm extending laterally from the intermediate mesoderm give rise to the somatic (parietal) and splanchnic (visceral) mesoderm. The somatic mesoderm is the more superficial layer, contributing to formation of the body wall. The splanchnic mesoderm, the deeper layer, remains adjacent to the endoderm and contributes to formation of the wall of internal organs in the embryo. The space enclosed by the somatic and splanchnic mesoderm is the coelom. Part of the coelom will be enclosed as the body cavity, while the remainder is transient. In embryos, all regions of the coelom are initially continuous.235 Complete isolation of the pericardial cavity from the common pleuroperitoneal cavity occurs initially in relation to growth of the common cardinal veins. Formation of the diaphragm, derived from many tissues, separates the pleural cavity from the peritoneal cavity. Malformations of the diaphragm may lead to congenital pleuroperitoneal or peritoneopericardial hernias (or both).235 Umbilical hernia results from a defect in development of the muscular wall around the umbilicus. Omphalocele is an embryonic herniation of abdominal contents through the umbilicus into the umbilical stalk. Complete absence of the ventral abdominal wall is most prevalent in cats.235 The abdomen extends from the diaphragm to the pelvis and contains the largest cavity in the body, the abdominal cavity.97 The abdominal cavity contains the alimentary canal; liver; pancreas; spleen; female reproductive tract; and many nerve plexuses, vessels, and lymph nodes. The diaphragm, the lumbar vertebrae and sublumbar muscles, the oblique and transverse abdominal muscles plus a small portion of the ilial wing on each side, and the rectus abdominus muscles bound the abdominal cavity.97 The abdomen can be divided by two transverse and two sagittal planes (Figure 86-1, A) into nine regions: the right and left hypochondriac regions, the epigastric or xyphoid region, the umbilical region, the right and left lateral regions (including the flanks and paralumbar fossae), the right and left inguinal regions, and the pubic region.97 Alternatively, the abdomen can be divided by one transverse and one sagittal plane (Figure 86-1, B) through the umbilicus into four quadrants: the right and left cranial quadrants and the right and left caudal quadrants. The quadrant divisions are most commonly used clinically. Three unpaired openings through the diaphragm lead into the abdominal cavity: the esophageal hiatus for passage of the esophagus, vagus trunk, and esophageal vessels; the caval hiatus for passage of the caudal vena cava; and the aortic hiatus for passage of the aorta, thoracic duct, azygous and hemizygous veins.97 Paired slitlike openings dorsal to the diaphragm and ventral to the psoas muscles separate the pleural and peritoneal cavities only by a thin layer of fused endothoracic and transversalis fascia. This area may be clinically significant in direct extension of certain disease processes between the pleural and abdominal cavities in the absence of defects in the diaphragm (pneumothorax/pneumoperitoneum, pyothorax/septic peritonitis, chylothorax/chylous ascites).97 The sympathetic trunk and splanchnic nerves also enter the abdominal cavity in this area.97 The abdominal and pelvic cavities communicate freely at the pelvic inlet. Other openings of the caudal abdomen include the inguinal canal on each side, allowing passage of the vaginal process with spermatic cord in the male and round ligament in the female, external pudendal vessels, and genital nerve; and the right and left vascular lacunae in the caudal abdomen containing the femoral artery and vein, lymphatics, and saphenous nerve.97 The linea alba extends from the xiphoid process to the pelvic symphysis.97 Cranial to the umbilicus, it is a wide midventral strip of collagenous tissue. Caudal to the umbilicus, the linea alba gradually narrows and thickens, finally blending with the prepubic tendon. The umbilicus in adults is a scar in the linea alba at the level of a transverse plane through the last ribs. The abdominal cavity in some animals has an incomplete mesodermal lining at the level of the umbilicus.97 A characteristic ring of subcutaneous hemorrhage around the umbilicus, referred to in human medicine as the Cullen sign, may appear in some cases of hemoperitoneum or peritonitis by direct extension from the abdominal cavity to the subcutis.78 The abdominal and pelvic cavities are lined by transversalis fascia with a covering of mesothelial cells, the peritoneum (Figure 86-2). The peritoneum and transversalis fascia are united by subserous areolar tissue, which contains large fat deposits in obese animals. Peritoneum lines the abdominal, scrotal, and pelvic cavities and covers the abdominal organs and their supportive structures. Parietal peritoneum lines the cavities, and visceral peritoneum covers the abdominal organs wholly or in part (Figure 86-3). The peritoneal cavity is a potential space between the visceral and parietal peritoneum and contains no organs except at the time of ovulation when an egg ruptures from the ovary. The peritoneal cavity is the largest preformed extravascular space in the body. Its surface area is approximately 150% of the total skin surface area.97 In common usage, the terms peritoneal cavity and abdominal cavity are interchangeable, but in strict anatomic terms, this usage is incorrect.97 Connecting peritoneum consists of double sheets of peritoneum between organs or connecting visceral to parietal peritoneum (Figure 86-4, A).97 These peritoneal folds comprise mesenteries, omenta, and ligaments (Figure 86-4, B). Mesenteries are wide serous folds by which vessels and nerves reach the organs. Ligaments attach organs to walls, such as the lateral ligaments of the liver, or attach organ to organ, such as the duodenocolic ligament.97 Additional peritoneal folds are associated with the urogenital organs. The broad ligaments in females and the genital folds in males, the lateral ligaments of the urinary bladder, and median ligament of the bladder are all peritoneal in origin.232 The greater omentum is composed of three portions, each consisting of a double peritoneal sheet.97 The largest portion is the bursal portion, attaching cranioventrally to most of the greater curvature of the stomach. The bursal portion extends caudally to the urinary bladder, covering the intestines. Folding of the greater omentum results in a superficial ventral layer (paries superficialis) and a deeper dorsal layer (paries profundus). The omental bursa is a potential space between these layers. The omental bursa is a closed sac except for its large opening, the epiploic foramen, bounded dorsally by the caudal vena cava and ventrally by the portal vein.97 The epiploic foramen has surgical significance. Bleeding from the liver can be temporarily arrested by placing a finger through the epiploic foramen just cranial to the pylorus and curling the finger ventrally to occlude the hepatic artery and portal vein.67 Migration of loops of small intestine through the epiploic foramen is rare but may result in intestinal strangulation.75 The bursal portion of the greater omentum is used surgically for omentalization of wounds and cavities, aiding in revascularization of tissues with impaired blood supply and in resolution of cysts, abscesses, and chronic wounds. The two remaining portions of the greater omentum are of less significance than the bursal portion. The splenic portion of the greater omentum extends to the hilus of the spleen to form the gastrosplenic ligament. The smallest portion of the greater omentum is the veil portion containing the left limb of the pancreas. The omentum contains aggregations of cells known as milky spots. Omental milky spots are a source of neutrophils, macrophages, and lymphocytes and are important components of peritoneal defense mechanisms.142 Organs that lie against the walls of the abdominal or pelvic cavities and that are covered on only one surface by peritoneum are considered retroperitoneal.97 Organs with a nearly complete covering of peritoneum that project freely into the abdominal, pelvic, and scrotal cavities are considered intraperitoneal. The kidneys, ureters for most of their length, and adrenal glands are commonly considered retroperitoneal. The aorta, caudal vena cava, and lumbar lymph nodes are also retroperitoneal.97 The peritoneum is a serous membrane composed of a single layer of squamous cells of mesothelial origin. The cell surface is covered with microvilli. The mesothelial cells are supported by a connective tissue stromal layer composed of elastic and collagen fibers, macrophages, lymphocytes, mast cells, glycosaminoglycans, and adipose cells.130 The peritoneum of the visceral surface of the diaphragm has fenestrations of the basement membrane, the stomata, of variable size depending on the species.10 Mesothelial cell processes regulate stomata size, increasing the size in peritonitis.142 In dogs and cats, these fenestrations are considered large, generally in the 4- to 16-µm diameter range or larger.10 These fenestrations and special lymphatic collecting vessels, the lacunae, are important in clearance of fluid and particles from the peritoneal cavity.203 The peritoneal cavity (i.e., the potential space between the visceral and parietal peritoneum) normally contains only a very small amount of peritoneal fluid to provide lubrication.178 Surfactant produced by peritoneal mesothelial cells acts as a lubricant.130 Normal juvenile animals appear to have a larger volume of peritoneal fluid present in the abdominal cavity (personal observation). The peritoneum is a bidirectional semipermeable membrane capable of both absorption and exudation or transudation.337 The peritoneum allows free exchange between peritoneal fluid and plasma, hence the efficacy of techniques such as peritoneal dialysis. Peritoneal fluid forms as a dialysate of plasma. There is constant production and absorption of peritoneal fluid. The colloid osmotic pressure of normal peritoneal fluid is approximately 28 mm Hg, largely the result of protein in the fluid. Normal peritoneal fluid protein is less than 3 g/dL (Table 86-1);130 however, a nonlinear relationship exists between protein concentration and colloid osmotic pressure.178 Normal peritoneal fluid lacks fibrinogen and does not clot. Antibacterial activity of normal peritoneal fluid is nonspecific, through complement and fibronectin. Normal peritoneal fluid is relatively acellular, containing less than 300 cells per mm3.142 Macrophages are the predominant cell type in normal peritoneal fluid, with small numbers of mesothelial cells and lymphocytes also present.142 In an early study of lymphatic drainage from the peritoneal cavity of dogs, finely particulate graphite could be observed delineating the lymphatics of the diaphragm 10 to 90 minutes after intraperitoneal injection.146 The primary route of drainage of particles was through the diaphragmatic lymphatics to the mediastinal lymph node and finally by way of the thoracic duct into the systemic circulation. Particles smaller than 10 µm in diameter (bacteria being 0.5 to 2.0 µm and red blood cells [RBCs] being 7 to 8 µm in diameter) are rapidly cleared from the peritoneal cavity by this mechanism. In cats, small particles in suspension injected intraperitoneally appear in the mediastinal lymph node within 3 minutes.81 In all species studied, particles cleared from the peritoneal cavity appear quickly in the systemic circulation and in the lungs. As a result, in bacterial peritonitis, bacteremia is an early and consistent finding. Movement of fluid from the peritoneal cavity into the diaphragmatic lacunae initially occurs with passive stretching of the diaphragm. Simultaneous diaphragmatic muscular contraction and decreased intrathoracic pressure during exhalation moves fluid through the lymphatics into efferent ducts along a pressure gradient.158 The right side of the diaphragm has a much greater distribution of lymphatics than the left side.146 The reason for this distribution is unclear. In addition to particle size, factors affecting particulate matter clearance include gravity, respiratory movements, diaphragmatic movement, intestinal activity, and intraperitoneal pressure.158 An experimental study in rats documented changes in lymph flow and lymph node drainage pattern after bowel resection, suggesting that lymphatic drainage of the peritoneal cavity is a complex and individualized process.246 Enlargement of sternal lymph nodes may signal peritoneal or retroperitoneal inflammation or neoplasia. In one young dog with mild pneumothorax and vague clinical signs, sternal lymph node enlargement prompted abdominal ultrasound examination, resulting in diagnosis of peritoneal disease, with a localized omental abscess containing a grass awn detected during exploratory surgery.155 There is a general cranial movement of fluid and particles within the peritoneal cavity toward the diaphragm. Intraperitoneal circulation is dynamic and spreads fluid and particulate matter throughout the peritoneal cavity (Figure 86-5). In humans, the pattern of intraperitoneal circulation and the locations of fluid accumulation have been related to common patterns of intraabdominal abscess formation.203 The exact pattern of intraperitoneal circulation and the speed of complete dispersion of material depend on the site of origin and the physical properties of the material in addition to the factors influencing clearance of particulate matter. Complete dispersion of oil-based contrast medium throughout the peritoneal cavity occurred at mean times of 19 minutes after cranial injection and 72 minutes after caudal injection in dogs.158 The speed of complete dispersion was significantly different between cranial and caudal injection sites in this study.158 Most of the contrast medium drained within 6 hours with open peritoneal drainage and within 24 to 48 hours with sump–Penrose drainage. The peritoneal cavity is estimated to be capable of absorbing fluid at a rate of 3% to 8% of body weight per hour.172 Intraabdominal pressure (IAP) can be measured indirectly through an indwelling transurethral urinary bladder catheter. It is a simple, inexpensive, and reportedly reliable technique.64,265 However, a recent prospective experimental study in horses showed only low to moderate correlation between direct and indirect methods of IAP measurements.226 The normal range for IAP in dogs is 2.0 to 7.5 cm H2O (mean, 4.50 ± 0.44 cm H2O).64 Increased IAP has been described experimentally and clinically.64,196 Increased IAP results from altered abdominal compartment compliance. The muscles of the abdominal wall and diaphragm as well as intraabdominal factors contribute to abdominal compartment compliance. Intraabdominal accumulations of gas, fluid, or tissue; multiple trauma; severe forms of acute pancreatitis; abdominal surgery; insufflation for laparoscopy; and external abdominal counterpressure elevate IAP.7,64 IAP is also increased in obesity and after massive fluid resuscitation.43 Acute IAP increase is associated with cardiovascular, respiratory, and abdominal organ dysfunction. IAPs of 15 mm Hg (20.4 cm H2O) in pigs induced by CO2 insufflation caused increased heart rate, increased mean arterial pressure, increased systemic vascular resistance, decreased cardiac output, decreased mesenteric arterial blood flow, decreased intestinal mucosal blood flow, and increased bacterial translocation.7 Marked increase in IAP has been referred to as acute abdominal compartment syndrome and is associated with multisystem organ failure and a poor prognosis in humans.7,306 Organ system dysfunctions described in acute abdominal compartment syndrome include acute pulmonary failure secondary to compressive atelectasis with increased peak inspiratory pressure and impaired gas exchange, increased intrathoracic pressure leading to increased pleural and pericardial pressures and decreased venous return to the heart, acute renal failure with marked oliguria, intestinal ischemia with resultant bacterial translocation, hepatic ischemia resulting in increased liver enzymes, increased intracranial pressure leading to decreased cerebral perfusion and neuronal injury, venous thrombosis and thromboembolism, and abdominal wall ischemia or necrosis.306 The clinical diagnosis of acute abdominal compartment syndrome in humans is made when high peak inspiratory pressure is accompanied by oliguria and an apparent tight abdomen.306 Urinary bladder pressure measurement greater than 20 to 25 cm H2O is suggestive; however, chronic elevation of IAP may be associated with obesity, pregnancy, and chronic ascites.306 In dogs, IAP was significantly increased for at least 24 hours after elective ovariohysterectomy and was also increased in 20 dogs with abdominal distension of various causes.64 Medical or surgical management may improve outcomes in animals with increased IAP. Surgical decompression should be considered when IAP nears 30 cm H2O, particularly in oliguric animals.64 In simulated intraabdominal hemorrhage in dogs, IAP elevations of 24 mm Hg (32.6 cm H2O) caused renal failure that remained reversible after 4 hours.196 Low-dose infusion of dobutamine (5 µg/kg/min) corrected intestinal mucosal hypoperfusion induced by moderately increased IAP (15 mm Hg or 20.4 cm H2O IAP) experimentally in pigs.7 Peritoneal mesothelium is easily injured but heals rapidly. Mesothelial cell loss occurs with exposure of the peritoneum to air or saline. Round cells of uncertain origin cover wounded mesothelium within 4 hours.158 These cells later resemble mesothelial cells. In rats, coverage of large areas of denuded peritoneum with cells resembling new mesothelial cells is complete by day 3.96 The exact origin of these new mesothelial cells is controversial. Differentiation from fibroblasts, mesothelial cell implantation from adjacent normal structures, and implantation of free-floating peritoneal macrophages have been proposed.96 Inflammatory cells and fibrin exude into the peritoneal cavity in response to surgical manipulations and many disease processes. In the absence of tissue ischemia, fibrinolysis occurs within 3 to 4 days, and adhesions do not form.95,129 However, when injury is accompanied by vascular damage, fibrin is infiltrated by fibroblasts producing collagen, which converts fibrinous adhesions to firm fibrous adhesions. In general, adhesions should be considered beneficial in that they bring additional blood supply to compromised tissue. However, adhesion formation may also contribute to significant morbidity, particularly in humans and horses, but also occasionally in small animals.19 The propensity for postoperative intraabdominal adhesion formation varies by species and many other factors. Adhesions to the laparotomy scar (Figure 86-6) are more likely to occur when peritoneum is sutured.63 In addition to ischemia, the likelihood of adhesion formation increases with endotoxemia, intestinal manipulation, bowel distension, and desiccation of serosal surfaces.129 Foreign body contamination of the peritoneal cavity during laparotomy from gauze fragments, lint, cotton fibers, glove powder, and antibiotic powder may result in granuloma formation and the development of fibrous adhesions.96 Latex, silicone, contrast agents, and vegetable matter have also been associated with adhesion formation.157 Meticulous operative technique reduces the likelihood of adhesion formation. Prevention of tissue desiccation, gentle tissue handling, meticulous hemostasis, precise suture placement, complete removal of blood clots and foreign debris, and thorough lavage are believed to minimize adhesion formation. Numerous physical and chemical manipulations to reduce intraabdominal adhesion formation have been reported, with some conflicting results. In horses, postoperative peritoneal lavage with lactated Ringer’s solution,129 intraoperative and postoperative administration of heparin,243 and hyaluronate–carboxymethylcellulose membrane implantation223 significantly reduce adhesion formation. In laboratory animals, intraperitoneal 0.4% hyaluronic acid solution,263 subcutaneous administration of TNP-470,55 and intraabdominal administration of fibrinolytic agents significantly reduce adhesion formation. Many other agents, including broad-spectrum antibiotics, corticosteroids, nonsteroidal antiinflammatory drugs (NSAIDs), dimethyl sulfoxide, dextrans, and interleukin-10, have been advocated to minimize adhesion formation.223 Physical techniques, including omentectomy and physical barriers, have also been advocated.55,182 Pharmacologic and physical techniques to minimize adhesion formation are rarely required in small animals. Figure 86-7 provides an overview of the pathophysiologic processes in peritonitis. Peritoneal defenses rely largely on innate immune mechanisms together with mechanisms to absorb and localize infection. Peritoneal fluid has innate antibacterial activity mediated by the complement system.142 Specifically, release of C3a and C5a stimulates neutrophil chemotaxis and degranulation of basophils and mast cells. Other innate defenses include absorption of bacteria through diaphragmatic lymphatics, phagocytosis by resident leukocytes and macrophages, abscess formation, and activity of resident natural killer cells.142 The specific immune system, mediated by lymphocyte activity, provides a secondary amplification system in response, particularly to intraabdominal sepsis. Peritoneum-associated lymphoid tissue and omental lymphoid tissue are important sites of immunoglobulin production.142 Peritoneal injury or contamination elicits an intense inflammatory reaction. There is an initial influx of protein-rich fluid from the vascular space accompanied by macrophages and neutrophils. There is activation of humoral opsonins, antibodies, and complement. Complement is involved in enhancement of the inflammatory response, opsonization of organisms, clearance of immune complexes, and cell lysis.130 Subendothelial mast cell degranulation releases vasoactive substances, increasing vascular permeability. Mast cell degranulation also releases complement and opsonins, with complement components chemotactic for macrophages and opsonins coating bacteria and promoting phagocytosis.142 Peritoneal-associated lymphoid tissues produce immunoglobulins. An intense interaction among peritoneal mesothelial cells, neutrophils, macrophages, mast cells, and lymphocytes via chemical signals that both loop and cascade results in additional cell recruitment and release of cytokines. Peritoneal mesothelial cells produce high levels of interleukin-8 (IL-8) in response to macrophage-derived tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β), augmenting neutrophil emigration by chemotaxis.130 The acute phase response is characterized by cellular and cytokine cascades initiated by IL-1β and TNF-α. Lymphocyte-derived cytokines, TNF-α, and IL-6 augment macrophage cytokine production during gram-negative peritonitis. Arachidonic acid metabolism in peritoneal mesothelial cells is stimulated by IL-1β and TNF-α, leading to increased prostaglandin synthesis.130 Interleukin-10 (IL-10) attenuates production of proinflammatory mediators and protects against development of lethal shock.219 Clinical manifestations of septic shock may be attributable to an imbalance between pro- and antiinflammatory cytokines.214,219 The systemic inflammatory response syndrome (SIRS), as described above, occurs commonly in animals and humans with peritoneal inflammation. The severity of the clinical response is proportional to the degree of elevation of proinflammatory cytokines, particularly TNF-α, IL-8, and IL-6.171 In a primate model of peritonitis, persistent elevation of IL-6 was a poor prognostic sign, with a mortality rate of 42%.171 Histamine and prostaglandin released by mast cells and macrophages cause vasodilation and increased permeability of peritoneal capillaries, leading to copious exudation of fluid rich in complement, immunoglobulins, clotting factors, and fibrin into the peritoneal cavity. The peritoneal fibrinolytic system is inactivated in inflammation. Lack of fibrin clearance leads to fibrin clumps, which can occlude peritoneal stomata and impede clearance of fluid and particles. Fibrin deposits sequester microorganisms from normal host defenses.186 The presence of fibrin in the peritoneal cavity is the first step in the process of fibrous adhesion formation. A number of intraperitoneal substances are known adjuvants in peritonitis.143 These substances either worsen the local and systemic inflammatory response or worsen the prognosis in bacterial peritonitis. Known adjuvants include gastric mucin, bile salts, hemoglobin, and barium. Gastric mucin polysaccharide has a heparin-like anticomplement effect, which inhibits phagocytosis.143 Bile salts lower surface tension, altering cell adhesion and lysing RBCs, releasing hemoglobin.143,326 Hemoglobin interferes with phagocytic cell chemotaxis, phagocytosis, and intracellular killing.143 Hemoglobin may also play an adjuvant role by providing the iron required by some microorganisms.186 Hemoglobin also inhibits bacterial clearance from the peritoneal cavity by interfering with lymphatic clearance mechanisms.139,186 Peritoneal fluid volume has an adjuvant effect in experimental peritonitis.91 Increased bacterial proliferation, slowed bacterial clearance, and increased mortality rates were observed with intraperitoneal injection of inoculum of identical bacterial numbers in increasing volumes of sterile saline.91 The systemic manifestations of peritoneal inflammation vary with the cause of peritonitis. In most cases, hypovolemia and hypoproteinemia result from movement of protein-rich fluid from the vascular space to the peritoneal cavity. Sequestration of fluid within the bowel lumen secondary to reflex ileus exacerbates hypovolemia. Respiratory acidosis and hypoxemia result from reflex diaphragmatic rigidity and increased IAP, impeding ventilation. Increased IAP also exacerbates hypovolemia by reducing cardiac venous return and cardiac output. Hypovolemia leads to hypotension and impaired organ perfusion, leading to metabolic acidosis and tissue hypoxia. Impaired renal perfusion leads to renal insufficiency that is compounded by decreased renal clearance of toxins, resulting in acute renal failure. Release of myocardial depressant factor from hypoxic pancreas exacerbates the cardiovascular alterations. A severe catabolic state results from a 25% increase in metabolic rate coupled with massive protein loss into the peritoneal cavity.157,287 Intrahepatic cholestasis has been described in dogs with severe extrahepatic bacterial infections, including peritonitis.310 Alteration of normal bile formation and flow is the apparent pathophysiologic mechanism resulting in icterus, increased liver enzymes, and increased bile acids. In canine models of septic shock, adrenergic stimulation increases intestinal venous pressure, injuring the mucosal barrier of the gut and promoting translocation of gut bacterial flora across the intestinal wall.121 In peritonitis associated with sepsis or endotoxemia, profound metabolic alterations associated with these conditions may be additive or synergistic. In models of sepsis, increased plasma insulin, glucagon, and catecholamines are associated with a hyperdynamic state.287 Oxygen consumption is markedly increased in sepsis.148 Endotoxin augments the inflammatory response in peritonitis by activation of complement, activation of arachidonic acid metabolism with resultant prostaglandin release and leukotriene production, and stimulation of macrophages, leading to further elevation of proinflammatory cytokine levels.111 Dogs and cats, however, are considered relatively endotoxin resistant.254 Exoenzymes of anaerobic bacteria commonly involved in septic peritonitis promote tissue invasiveness and cause inflammation, necrosis, and suppuration. Capsular polysaccharides of anaerobic bacteria promote adhesion to peritoneal surfaces and abscess formation.111 Fever is a nonspecific acute-phase response that appears to be largely beneficial in patients with infections, improving survival and shortening duration of illness.163 Experimentally, bacterial proliferation is profoundly reduced in febrile animals.163 When housed at increased ambient temperatures, survival rates in mice inoculated intraperitoneally with a lethal strain of Klebsiella pneumoniae increased from 0% to 50%.163 The mechanisms of the beneficial effects of fever are unclear but appear to relate to enhanced host defenses through enhancement of cytokine expression, particularly TNF-α and IL-1β. Pharmacologic suppression of an animal’s febrile response may be deleterious. In humans, peritonitis is the leading cause of multisystem organ failure.301 Organ systems that can be affected in patients with multiple organ dysfunction syndrome (MODS) include the cardiovascular, respiratory, renal, hepatic, hematologic, gastrointestinal, endocrine, and immune systems. Injury to distant organ systems in patients with peritonitis is postulated to be mediated primarily by neutrophils.301 MODS has recently been reported in a large, multicenter, retrospective series of dogs treated surgically for septic peritonitis secondary to gastrointestinal tract leakage.170 Fifty percent of dogs in this study had MODS. The mortality rate was 70% for dogs with MODS and 25% for those without MODS.170 Omentum participates actively in the body’s response to peritoneal injury or contamination. Omentum helps isolate and seal the source of contamination by formation of omental adhesions in response to fibrinous exudate. The omentum also absorbs bacteria and other particulate matter; it is the only organ in addition to the peritoneum capable of doing so. The omentum brings a rich blood supply, high absorptive capacity, and pronounced angiogenic activity, playing a central role in peritoneal defenses.130 Although the omentum, when present, plays an important role in particle clearance, omentectomy has no effect on clearance of particulate matter from the peritoneal cavity.143 Peritoneal inflammation induces gastrointestinal ileus by sympathoadrenergic reflex inhibition.118 These inhibitory reflexes completely block myenteric cholinergic neurons, but this response is eliminated during spinal anesthesia and adrenergic blockade.118 Ileus is an early and consistent finding in peritonitis, although dogs are considered relatively resistant to paralytic ileus.217 In dogs, altered intestinal motility has occurred in response to intraperitoneal injection of gastric juice, chemical irritation, mechanical peritoneal irritation, thermal peritoneal irritation, gastric perforation, and “appendiceal” ligation, but there was no effect of retroperitoneal blood administration and partial occlusion of the cranial mesenteric artery.217 In a dog model of acute peritonitis by “appendiceal” ligation, spike activity was significantly decreased by 3 hours, motility was inhibited by 6 hours, and complete adynamic ileus was established by 9 hours.2 Other factors commonly encountered in animals with peritonitis may also predispose to adynamic ileus. These include ischemia, chronic bowel distension, endotoxemia, electrolyte imbalances, and the effects of anesthesia.320 Peritoneal irritation causes reflex rigidity of the abdominal and diaphragmatic muscles. Diaphragmatic rigidity impedes respiratory movements. Diaphragmatic rigidity and decreased respiratory movement reduce intraperitoneal circulation and decrease lymphatic clearance of fluid and particulate matter through the diaphragm.203 Although abdominal pain with splinting of the abdominal wall muscles may be observed in some cases of peritonitis, this commonly described physical finding is often absent.150 In two recent retrospective studies of septic peritonitis in cats, signs of pain during abdominal palpation were reported in only 38% to 64%.66,245 The peritoneum is innervated by somatic nerves, with pain from peritoneal inflammation localized to the site of inflammation.207 The abdominal viscera are innervated by visceral nerves that respond primarily to stretch. Visceral pain is poorly localized and maps to areas reflecting the embryologic development of the gastrointestinal tract.207 These features may contribute to poorly localizable and vague signs of abdominal pain in animals with peritonitis. Primary peritonitis is spontaneous inflammation of the peritoneum in the absence of an evident intraabdominal source of infection or history of penetrating peritoneal injury.80,186 In humans, primary peritonitis generally has a bacterial cause and is also referred to as spontaneous bacterial peritonitis.186 In contrast to secondary peritonitis, primary peritonitis is monobacterial in most cases. Primary peritonitis in humans is most often associated with alcoholic cirrhosis and ascites but has also been associated with nephrotic syndrome, acute viral hepatitis, malignancy, and systemic lupus erythematosus. The route of infection has been postulated to be primarily hematogenous, but bacterial translocation across the intact intestinal mucosa and bacteremia in portosystemic shunting have also been implicated.186 The most common causative organisms in cirrhotic humans are Escherichia coli and Klebsiella spp., with mortality rates of 50% to 70% at 1 year.179 Impaired host defenses contribute to the predisposition to primary peritonitis. Destruction of bloodborne bacteria by the hepatic reticuloendothelial system is impaired in experimental cirrhosis and alcoholic liver disease.164 Alcoholic cirrhotic patients also have impaired neutrophil function and hypocomplementemia.186 Bactericidal and opsonic activity of normal peritoneal fluid is impaired in ascites.186 The best example of primary peritonitis in small animals is feline corona virus infection resulting in feline infectious peritonitis in cats.284 The precise route of entry of the virus in cats is not known, but in utero transmission suggests a hematogenous route of infection in the fetus.248 Feline infectious peritonitis should always be considered as a differential diagnosis in cats with peritoneal effusions and abdominal masses.138,173,307,342 A recent retrospective study of primary bacterial peritonitis in 15 dogs and nine cats over a 16-year period revealed monobacterial infection in 56% of canine and 100% of feline cases.80 Eighty percent of the bacteria cultured in dogs and 60% of bacteria cultured in cats in this study were gram-positive organisms.80 Seven of 15 dogs (46.6%) and four of nine cats (44.4%) survived to discharge from the hospital. Three earlier cases of apparent spontaneous peritonitis, which could be considered primary peritonitis, have been reported in small animals.57,85,162 An 18-week-old kitten that died from acute peritonitis had Salmonella typhimurium isolated in pure culture. Although ingestion of the organism was suspected, there was no evidence of enteritis.162 In an adult cat with clinical signs suggestive of bacterial peritonitis, Chlamydia psittaci was isolated from the peritoneal exudate.85 Septic peritonitis caused by Clostridium limosum was diagnosed in a dog with a mast cell tumor.57 Compromised immunocompetency would be suspected in these animals. One dog with suspected neoplastic peritonitis on the basis of abdominal ultrasound findings had Mycobacterium microti diagnosed at necropsy, with the source of infection unknown.83 Citrobacter freundii was isolated in pure culture from two dogs with fibrinous peritonitis.110 This was considered an opportunistic pathogen in immunoincompetent animals, most likely of gastrointestinal origin. Blastomyces dermatidis recovered from the peritoneal cavity by diagnostic peritoneal lavage has been reported for the first time in a dog.233 Other fungal causes of presumed primary peritonitis include histoplasmosis and candidiasis in addition to sporadic reports of other opportunistic organisms.290 Secondary generalized septic peritonitis is the most common form of peritonitis in dogs.156 Secondary peritonitis most commonly results from loss of integrity of the gastrointestinal tract and direct inoculation of the peritoneal cavity with endogenous bacterial flora.156 Secondary peritonitis is an important clinical condition in dogs and cats, with a high mortality rate. In one study, 85% of dogs that developed intestinal anastomotic leakage died despite aggressive treatment.260 There are also many other causes of secondary peritonitis, both aseptic and septic (Box 86-1). Immunocompetency and host defense mechanisms are generally considered normal in secondary peritonitis.186 Animal models of generalized aseptic peritonitis have been created by mechanical abrasion of the parietal peritoneum or serosal surfaces of abdominal organs.278 A mild inflammatory reaction occurs in response to exposure of the mesothelial cells of the peritoneum to air. Thus, surgical exposure of the peritoneal cavity and insufflation for laparoscopy result in mild inflammation. More intense inflammatory reaction is elicited by the presence of foreign materials such as sutures, lint, hair, vegetable matter, and surgical swabs. The intensity of inflammation is related to reactivity of the material, particle size, volume of foreign material, and presence of bacteria. Inert materials such as stainless steel and titanium can be present for years in the peritoneal cavity with little or no inflammatory response. Natural fibers such as cotton, silk, and linen elicit intense inflammatory reactions. Smooth materials tend to elicit a lesser inflammatory response than irregular or woven materials. Retained surgical sponges are common intraperitoneal foreign bodies. Surgical sponges with radiopaque markers are easily identified on radiographs (Figure 86-8). The diagnosis of retained intraperitoneal surgical sponges without radiopaque markers is more challenging. Clinical signs are usually nonspecific, with an abdominal mass or draining sinus in an animal with a history of previous surgery being suspicious. Ultrasonography and ultrasound-guided fine needle aspiration may assist diagnosis.205,215 Surgical swab granuloma is sonographically characterized as a hypoechoic mass with an irregular hyperechoic center.215 Cytology may reveal mononuclear inflammatory cells and multinucleate giant cell along with fibers. The combination of survey abdominal radiographs and sonography enabled detection of retained surgical sponges in six of seven dogs (86%) in one study.215 During celiotomies for any reason, all surgical sponges should be accounted for before abdominal closure by comparison of initial and final sponge counts. Granulomatous peritonitis and severe adhesion formation have long been recognized as important sequelae to contamination of the peritoneal cavity with surgical glove powder in some individuals. These reactions were initially considered foreign body reactions to the powders used as glove lubricants. The frequency of glove powder–related granulomatous peritonitis has diminished over time with changes in glove powders and manufacturing processes. Talcum powder contamination of the peritoneal cavity has been associated with granulomatous peritonitis, localized peritoneal granulomas, fecal fistulas, sinus formation, intestinal obstruction, delayed wound healing, and even death.93 Endogenous chemical contamination of the peritoneal cavity may occur from gastric fluid, bile, pancreatic enzymes, or urine. Of these, bile and urine produce little peritoneal inflammation unless they are initially or subsequently infected with bacteria.3,65,137 Peritoneal irritation with gastric fluid is most likely related to its acidity. Autodigestion by pancreatic enzymes causes intense peritoneal inflammation (Figure 86-9).
Peritoneum and Retroperitoneum
Embryology
Anatomy
Natural Openings
Umbilicus and Linea Alba
Transverse Fascia, Peritoneum, and Peritoneal Cavity
Omentum
Retroperitoneum
Microscopic Anatomy
Physiology
Lymphatic Drainage
Intraperitoneal Circulation
Intraabdominal Pressure
Healing of Peritoneal Injury
Adhesion Formation
Adhesion Prevention
Pathophysiology
Peritoneal Defenses
Inflammatory Response
Omentum
Ileus
Reflex Rigidity
Peritonitis Classifications
Primary Peritonitis
Secondary Peritonitis
Aseptic Peritonitis
Mechanical and Foreign Body Peritonitis
Starch Granulomatous Peritonitis61,238
Chemical Peritonitis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Peritoneum and Retroperitoneum
Only gold members can continue reading. Log In or Register to continue