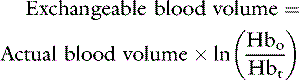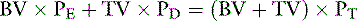Chapter 17 Perioperative Management of Fluid Therapy
1. To establish and maintain venous access. A minimal rate of fluid administration is necessary (e.g., 3 mL/hr) and will ensure rapid access to the circulation in the event of an emergency in the perioperative period.
2. To counter the physiologic changes associated with anesthetics. Most of the drugs and techniques used to anesthetize animals have some effect on the circulation.
3. To replace fluids lost during anesthesia and surgery. During the procedure, the animal cannot drink and its metabolic rate is reduced (decreased production of metabolic water). At the same time, the animal continues to produce urine, salivate, secrete fluid into the gastrointestinal tract, and lose water by evaporation from the respiratory tract. The aim should be at least to replace the expected insensible fluid losses.
4. To correct fluid losses caused by disease and replace ongoing losses attributed to the procedure. The volume of fluid lost or gained depends on the type of surgical procedure, the skill of the surgeon, the preoperative state of the animal, and the equipment used by the anesthetist. Trauma and surgery are associated with increased secretion of vasopressin, and additional secretion may occur as a result of hypotension or hypovolemia. Other stress hormones (e.g., cortisol, catecholamines, renin) released during the procedure also may play a role in upsetting normal fluid homeostasis and warrant perioperative fluid therapy.
Changes in vascular volume
Hypovolemia
Hypovolemia may be caused by fluid loss directly from the vascular space (e.g., hemorrhage), a more general loss (e.g., dehydration), or changes in vascular tone. In all cases, fluid should be given to replace the loss. For a simple loss in which the composition of the vascular space is relatively normal, the loss can be replaced effectively using an isotonic crystalloid, a hypertonic crystalloid, an artificial colloid, or a blood product. The fluid used depends on the severity of the loss and the financial resources of the client. Acute blood loss of up to 30% of blood volume can be replaced adequately using a crystalloid solution (assuming normal hematocrit and total protein concentration before therapy), whereas a loss of 50% of blood volume or more will probably require blood component therapy and possibly additional crystalloid or colloid support. Occasionally, fluid therapy is not sufficient, and surgical management is required to stop bleeding (e.g., penetrating trauma with major blood vessel rupture). In these instances, it is crucial to have one or more large-gauge venous catheters in place in an attempt to keep pace with the loss. In cats and dogs weighing less than 5 kg, it usually is feasible to place an 18-gauge catheter in the jugular vein. In many dogs of 5 to 15 kg, it is feasible to place a 16-gauge catheter in a cephalic vein, whereas in dogs more than 15 kg, a 14-gauge catheter normally may be placed. After the catheter has been placed, the animal should be anesthetized using a technique that induces minimal disturbances in volume status and cardiovascular function. Some investigators advocate withholding fluids from trauma patients with major vessel rupture before surgical intervention. In one study of human patients, a marginal benefit was demonstrated using this approach.14 Others have advocated resuscitation to lower than normal blood pressures to minimize the chance of dislodging a fragile clot or increasing the rate of hemorrhage.55 It is likely to be a realistic approach only when blood loss is rapid and surgery can be performed immediately.157 In patients with major blood loss but no central vessel rupture, it is more appropriate to replace the volume deficit before anesthetizing the animal.
If the patient is expected to lose a large volume of blood during an anticipated elective surgery, the animal can donate blood in advance so as to have autologous blood available. The animal can donate 1 unit of blood and then return 3 weeks later, at which time the first unit of blood can be returned to the animal and 2 units of blood drawn. This procedure can be repeated to collect several units from the same animal. This approach usually is not possible because of the lead time needed to complete these multiple collections, but a single donation technique has been reported for cats undergoing partial craniectomy.64 Another alternative in an animal with relatively normal hematocrit and total protein concentration is to use acute normovolemic hemodilution, collect blood immediately before surgery, and replace it with three times the volume of crystalloid or the same volume of colloid. The expectation is that the animal will lose less protein and red cell volume during the surgery because of hemodilution, and the collected blood will be available for transfusion when it is needed after surgery. The formula for calculating the hemodilution was originally described by Bourke and Smith20:
where Hb0 is the original hemoglobin concentration, and Hbt is the target value. This formula tends to overestimate the exchangeable blood volume, and a more accurate iterative formula has been published that uses a more sophisticated calculation technique.121
Although these techniques may be beneficial under special circumstances, they have been evaluated in human medicine and have been found to be very expensive and to provide little benefit to the patient.21,37,73,100,163 Nevertheless, the American Society of Anesthesiologists (ASA) published guidelines for the use of packed red cells that include the following statements:143
1. When appropriate, preoperative autologous blood donation, intraoperative and postoperative blood recovery, acute normovolemic hemodilution, and measures to decrease blood loss (deliberate hypotension and pharmacologic agents) may be beneficial.
2. The indications for transfusion of autologous red blood cells (RBCs) may be more liberal than for allogeneic RBCs because of the lower (but still significant) risks associated with the former.
Changes in content
Anemia
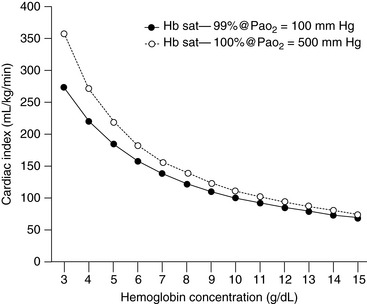
The major concern with anemic patients is the supply of oxygen to the tissues after the animal has been anesthetized with drugs that may impair cardiovascular function. In the chronically anemic animal, some compensation already has occurred to facilitate delivery of oxygen to the tissues. This compensation usually occurs as a result of an increase in cardiac output and a change in the affinity of hemoglobin for oxygen. When the animal is anesthetized, especially using drugs such as α2 agonists or inhalants, cardiac output is decreased, which reduces the delivery of oxygen to the tissues. Administration of 100% oxygen increases the amount of oxygen in solution (0.3 mL per 100 mL of blood per 100 mm Hg pressure), but this effect provides little compensation for the decline in cardiac output. Figure 17-1 illustrates the relationship between hemoglobin concentration and the cardiac index assuming a constant saturation of hemoglobin (99%) to deliver oxygen at a given rate (15 mL/kg/min). The second line shows the same relationship for a Pao2 of 500 mm Hg assuming a hemoglobin saturation of 100%. In acute anemia, the animal may have been able to increase cardiac output, but there has not been sufficient time for changes in hemoglobin affinity to occur, and the delivery of oxygen is likely to be decreased further. What is a “critical” hemoglobin concentration? In many experiments, carried out in dogs, the critical hemoglobin concentration is defined as the point at which oxygen delivery fails to keep up with tissue oxygen demand. In the healthy, lightly anesthetized dog, this concentration appears to be approximately 3 g/dL but varies with the anesthetic used and increases substantially at deeper planes of anesthesia.164,166 Many human patients are anesthetized and survive with hemoglobin concentrations as low as 3 to 4 g/dL, but anesthesia is not recommended in this situation unless great care is taken to ensure that the patient has adequate cardiovascular reserve and unless techniques can be used that minimize reduction in cardiac output.8,32 The ASA guidelines are based on the available literature in human medicine.143 The ASA recommendations for use of packed red cells include the following:
1. Transfusion is rarely indicated when the hemoglobin is greater than 10 g/dL and is almost always indicated when it is less than 6 g/dL, especially when the anemia is acute.
2. The determination of whether intermediate hemoglobin concentrations (6 to 10 g/dL) justify or require RBC transfusion should be based on the patient’s risk for complications of inadequate oxygenation.
3. The use of a single hemoglobin “trigger” for all patients and other approaches that fail to consider all important physiologic and surgical factors affecting oxygenation are not recommended.
A review in the Cochrane database examined the use of restrictive versus liberal transfusion practices and could identify no adverse effects of the use of transfusion triggers in the 7 to 9 g/dL range.84 Although hemoglobin concentration is reported on the complete blood count, it is more common for veterinarians to evaluate the hematocrit, which usually is approximately three times the hemoglobin concentration (expressed in g/dL). A scoring system for the rational use of packed RBCs in dogs was developed in an attempt to decrease unnecessary use.102 However, this scoring system did not account for blood transfusions under conditions of rapid blood loss and failure to maintain blood pressure. It is important to assess anemic dogs and cats carefully and to estimate the likelihood of blood loss during the procedure. A dog with a hematocrit of 18% and a healthy cardiovascular system about to undergo a noninvasive diagnostic procedure may be a candidate for anesthesia without previous transfusion. A patient with the same hematocrit but with clinically relevant mitral regurgitation and about to undergo an exploratory laparotomy for an undefined abdominal mass would be more likely to require a preoperative blood transfusion.
Polycythemia
Patients with polycythemia are at risk for complications because of the increased viscosity of their blood. High viscosity increases myocardial work and may lead to inadequate flow in some capillary beds, especially if the animal becomes hypotensive.9 The hematocrit should be reduced to at least 65% by removal of blood and replacement with an isotonic crystalloid before the polycythemic patient is anesthetized. Animals with polycythemia caused by chronic hypoxia (e.g., tetralogy of Fallot) must be monitored carefully for signs of inadequate oxygen delivery when such hemodilution is undertaken.
Hypoproteinemia
Hypoproteinemia also may affect the balance between hydrostatic and colloid osmotic pressure, leading to increased loss of fluid from the capillaries. This effect is of particular concern to the anesthetist because it may increase the likelihood of pulmonary edema formation. Total plasma protein and colloid osmotic pressure typically decrease during anesthesia in dogs whether or not fluids are administered.52,198 Clinically, this effect is of limited importance unless there is a strong possibility that left atrial pressure is increased (e.g., low oncotic pressure in an animal with mitral regurgitation).
Hyponatremia
Rapid correction of hyponatremia may be necessary to treat cerebral edema (usually only when serum sodium is <130 mEq/L). With acute hyponatremia, rapid correction may not cause any complications in the brain, but with chronic hyponatremia, a rapid change in serum sodium concentration can lead to an osmotic demyelination syndrome or myelinolysis occurring one to several days after therapy.111,131 In both acute and chronic situations, the rate of change should be approximately 0.5 to 1 mEq/hr unless the patient is manifesting signs of cerebral edema, in which case initial therapy with 3% saline may be used to increase serum sodium concentration by 5 to 6 mEq/L over 2 to 3 hours. Ideally, hyponatremia should be corrected before surgery; however, given the required time frame, this is not always possible. Therefore the anesthetist must be prepared to monitor changes in serum sodium concentration carefully to prevent myelinolysis. It may be necessary to administer a diuretic to facilitate excretion of free water (see Chapter 3).
Hypernatremia
Rapid correction of hypernatremia can lead to acute cerebral edema. If the patient is severely hypovolemic, it is important to correct that deficit using a solution with a sodium concentration similar to that of the patient. If the animal is not severely dehydrated and the serum sodium exceeds 165 mEq/L, correction should proceed slowly to achieve a rate of change of 0.5 to 1 mEq/hr using 0.45% NaCl or 5% dextrose. In dogs, administration of 5% dextrose at 3.7 mL/kg/hr should decrease the serum sodium concentration by 1 mEq/hr. Hypernatremia may increase the minimum alveolar concentration of inhalants, and a higher dose may be required to maintain anesthesia.180
Hypokalemia
Hypokalemia can lead to muscle weakness, cardiac arrhythmias, hypotension, and renal insufficiency with associated metabolic acidosis in dogs and cats. In patients with mild hypokalemia but no clinical signs and no identifiable underlying cause, it probably is unnecessary to treat the animal. The patient with hypokalemia that is likely to have a whole-body deficit of potassium should be treated to correct this deficit if possible. The usual recommendation is to correct the deficit at a maximal rate of 0.5 mEq/kg/hr, although higher rates can be used if a severe deficit of total body potassium is suspected (up to 1.0 mEq/kg/hr). If the hypokalemic patient must be anesthetized, it is important to monitor for cardiac arrhythmias and to recognize that the heart will be refractory to class I antiarrhythmic drugs (e.g., quinidine, procainamide, lidocaine) and more sensitive to the toxic effects of digitalis glycosides. Hypotension may occur because there is a decrease in systemic vascular resistance possibly related to decreased sensitivity to angiotensin II.66 The pressor response to norepinephrine is normal. If muscle relaxants are to be used, it is prudent to start with a dose that is 30% to 50% lower than the normal dose and titrate the final dose to effect. Care should be taken administering glucose, sodium bicarbonate, or β2-agonists because they tend to decrease serum potassium concentration. If a potassium-supplemented solution is to be used during anesthesia to correct the deficit, it should be used in conjunction with a solution containing a normal concentration of potassium (4 to 5 mEq/L), and the two solutions should be clearly labeled. If the animal requires a bolus of fluid during anesthesia, the solution with normal potassium concentration should be used, thus reducing the risk of iatrogenic hyperkalemia. Solutions containing more than 60 mEq/L of potassium should be given via a central vein.
Hyperkalemia
Hyperkalemia also is associated with muscle weakness and cardiac arrhythmias. If these signs are present, it is crucial to reduce the effects of hyperkalemia even though it is not possible to reduce total body potassium content without treating the primary condition (e.g., oliguric renal failure, urethral obstruction). Animals with moderate hyperkalemia (6 to 7 mEq/L) are more likely to develop arrhythmias during anesthesia even if they have not demonstrated electrocardiographic abnormalities earlier. Therapy for hyperkalemia includes administration of calcium to alter the threshold potential of cells, sodium bicarbonate to alter the flux of potassium across the cell membrane, and glucose to facilitate movement of potassium into cells. Insulin may be used with glucose to prevent hyperglycemia, but the blood glucose concentration must be monitored for several hours to avoid hypoglycemia. β-Adrenergic agonists such as albuterol and salbutamol have been used to manage hyperkalemia, and their activity may be enhanced with the use of insulin.6,113 One study in dogs documented the effect of epinephrine and ritodrine in reducing hyperkalemia.61 After the animal is anesthetized, ventilation should be monitored and controlled if necessary because hypercapnia may decrease pH and facilitate potassium efflux from cells. Depolarizing muscle relaxants (e.g., succinylcholine) should be avoided because they may cause release of potassium from cells. Nondepolarizing relaxants should be used cautiously (50% to 70% of the normal dose) to prevent prolonged effects. The patient should be monitored carefully by electrocardiography and frequent measurements of serum glucose, potassium, and ionized calcium concentrations and acid-base status (see Chapter 5).
Hypercalcemia
Signs of muscle weakness also may be seen with hypercalcemia, but arrhythmias are relatively uncommon. When they do occur, cardiovascular manifestations include bradycardia with prolonged PR–interval, wide QRS complex, and shortened QT–interval. Hypercalcemia is difficult to treat acutely and usually requires treatment for at least 24 hours before anesthesia (see Chapter 6).
Hyperosmolality
Hyperosmolality usually is associated with hypernatremia, hyperglycemia, ketoacidosis, uremia, or the presence of exogenous toxins (e.g., ethylene glycol). In some cases, it may be impossible to reverse the hyperosmolar state adequately before anesthesia because therapy (e.g., hemodialysis) may require an invasive procedure. Hyperosmolality may be associated with disruption of the blood-brain barrier leading to greater uptake of some drugs.200 This is unlikely to affect most anesthetics because they readily cross the blood-brain barrier normally. The hyperosmolar state associated with hypernatremia may increase the dose of inhalant required for anesthesia.180
Hypoglycemia
Hypoglycemia in an awake patient usually is manifested by somnolence progressing to coma. In the anesthetized animal, there may be no outward signs, and unless blood glucose concentration is being monitored, it is unlikely that hypoglycemia would be detected. Hence, it is important to recognize and manage hypoglycemia preoperatively. Most animals regulate their blood glucose concentration closely, but this may not be the case in very young animals, those with insulinomas, and animals with portosystemic shunts. It usually is unnecessary to remove very young animals from their dam until the time of premedication if they are receiving a liquid diet only. If they have been orphaned or are ill and have not been taking in fluids, it is best to check blood glucose concentration before anesthesia and treat accordingly. If blood is difficult to obtain, the animal can be given some oral glucose in the form of Karo syrup (ACH Food Companies, Inc., Memphis, Tenn.) or some other clear dextrose-containing fluid.58 Intraoperatively, it may be best to use a 2.5% to 5% glucose solution intravenously. Postoperatively, these patients should be monitored carefully or given additional Karo syrup until they can return to their previous feeding regimen. Animals with insulinomas can have resting blood glucose concentrations of 30 to 40 mg/dL and may tolerate these low glucose concentrations quite well. If exogenous glucose is administered as a bolus to an animal with hyperinsulinism, massive release of insulin may trigger a hypoglycemic crisis. Therefore it is important to use relatively dilute solutions of glucose and administer them as an infusion rather than as a bolus. We typically administer 2.5% glucose to these patients the night before surgery at 1 to 1.5 times the normal maintenance rate. Intraoperatively, blood glucose concentration is monitored carefully, and glucose infusions are continued as necessary. After the tumor is removed, blood glucose concentration usually returns rapidly to the normal range. Animals with portosystemic shunts may become hypoglycemic, and glucose supplementation may be needed in the perioperative period. In one retrospective series, 2 of 13 dogs with portosystemic shunts were reported to have developed hypoglycemia intraoperatively.105 Postoperative administration of dexamethasone (0.1 to 0.2 mg/kg IV) may be helpful in managing hypoglycemia in these cases.87
Hyperglycemia
Hyperglycemia typically occurs in diabetic dogs and cats, and in stressed cats. Hyperglycemia itself may not be dangerous; however, if blood glucose concentration exceeds 400 mg/dL, it may contribute to a hyperosmolar diuresis with subsequent dehydration. With diabetic animals, it is ideal if anesthesia can be postponed until blood glucose concentration can be better regulated. If this is not feasible, the animal should be treated with insulin and glucose to stabilize blood glucose concentration between 200 and 300 mg/dL. In patients with brain trauma or those suffering from focal or global brain ischemia during surgery, hyperglycemia may be detrimental to the neurologic outcome.36,165,166,191 In animal models, blood glucose concentrations as low as 150 to 200 mg/dL have been shown to have negative effects on outcome, but the threshold for cerebral damage seems to be approximately 200 mg/dL.115,166 In a study of dogs, dextrose administration was associated with greater renal damage after an ischemic insult than lactated Ringer’s solution (LRS).128 It is thought that increased intracellular glucose contributes to lactic acidosis in the cell, decreasing the chance of cell survival.
Metabolic acidosis
Dogs and cats generally tolerate moderate acidosis reasonably well. However, severe acidosis is likely to lead to reduced activity of enzyme systems in the body with subsequent alterations in energy production and metabolism of drugs. Acidosis also may alter the activity of some anesthetic drugs because more of the un-ionized active form of anionic drugs is available at lower pH values. In patients with acidosis arising from insufficient oxygen delivery to tissues because of inadequate circulating volume, correction of the volume deficit may reverse acidosis without the need for further therapy. Dogs and cats with diabetic ketoacidosis rarely require exogenous alkali if fluid therapy and insulin administration are managed appropriately. In cases in which the underlying condition is difficult to reverse (e.g., hypoxemia related to airway pathology, heart failure, pheochromocytoma), it is important to manage the acidosis before anesthesia. This is normally done using sodium bicarbonate, but Carbicarb and tromethamine may also be used (see Chapter 10). Sodium bicarbonate usually is available as an 8.4% solution with 1 mEq bicarbonate per milliliter and an osmolality of 2000 mOsm/L. In animals that are hyperosmolar or hypernatremic, it may be advisable to dilute bicarbonate to an isosmotic solution to prevent further exacerbation of the animal’s condition. An osmolality of 300 mOsm/L can be achieved by diluting 1.5 mL of the 8.4% solution in 8.5 mL of sterile water. Sodium bicarbonate also should not be administered through the same intravenous line as catecholamines because it inactivates them (Table 17-1). Care should be taken when administering sodium bicarbonate to patients with respiratory depression because it increases the production of CO2. If the animal is unable to increase its ventilation in response to increased production of CO2, there may be little overall change in pH.
Table 17-1 Compatibility of Intravenous Solutions with Other Drugs That Might Be Administered During Anesthesia
| Solution | Comments |
|---|---|
| 5% Dextrose | The pH of the solution ranges from 3.5 to 6.5, so alkaline solutions may precipitate. |
| Lactated Ringer’s | Slightly acidic and contains calcium. Do not administer with blood products. Sodium bicarbonate may also react with the calcium and form calcium carbonate. |
| Acetated polyionic | If it contains no calcium, can be used with blood products and sodium bicarbonate. |
| Sodium chloride 0.9% | Usually slightly acidic but is compatible with most intravenous solutions; may cause precipitation if added to mannitol. |
| Sodium bicarbonate | Alkaline solution—incompatible with dobutamine, dopamine, isoproterenol, norepinephrine, and epinephrine. May react with calcium in solution (e.g., lactated Ringer’s, acetated Ringer’s, some polygelatins). |
| Dextrans | Slightly acidic—may degrade acid-labile drugs and may form drug complexes but appear to be compatible with most intravenous solutions. |
| Hetastarch | May be incompatible with some antibiotics—crystals formed with amikacin, cefamandole, cefoperazone, and tobramycin. |
| Polygelatins | Some preparations contain calcium, and these should not be used with blood products or sodium bicarbonate. |
| Blood and plasma | Do not administer through the same line as calcium salts. |
Metabolic alkalosis
Conditions that cause metabolic alkalosis may be associated with a high mortality rate, and 10 of 20 dogs with primary alkalemia died in one study.151 Induction of anesthesia in an alkalotic patient may be associated with an increased dose requirement because of a decreased amount of un-ionized drug. In most cases, management of metabolic alkalosis requires the administration of chloride-containing solutions. This normally is achieved using 0.9% NaCl supplemented with KCl. Mild alkalosis may be caused by hypoalbuminemia, and correction of serum albumin concentration may be sufficient to correct the alkalosis.
Changes in distribution
Dehydration
Dehydration reduces vascular volume and results in changes in the volume of the intracellular space. The type and extent of change in the various compartments depend on the type of fluid lost. With pure water loss, volume contraction occurs in the intracellular compartment, whereas with hypotonic dehydration, an increase in the volume of the intracellular compartment may occur. With pure water loss, it is relatively simple to replace the circulating volume, but it takes longer to replenish the volume lost from the rest of the body. These concepts are discussed further in Chapter 3.
Pulmonary edema
A decrease in colloid osmotic pressure rarely causes pulmonary edema acutely in dogs and cats, but it is important to take low colloid osmotic pressure into account when designing an anesthetic regimen because pulmonary edema may occur with smaller increases in pulmonary hydrostatic pressure. Both ketamine and large doses of oxymorphone have been shown to increase pulmonary vascular pressures.42,82 If it is thought that low colloid osmotic pressure is contributing to pulmonary edema, therapy should be instituted to increase colloid osmotic pressure (e.g., plasma, dextrans, hetastarch [HES], polygelatins). In the case of pulmonary edema related to leaky membranes, therapy should be aimed at reducing pulmonary vascular pressure (e.g., nitroprusside, diuretics) and providing supportive care for the animal. Supportive care involves provision of oxygen, suction of froth from the airway, and institution of positive-pressure ventilation if necessary. Mechanical ventilation may improve gas exchange in patients with pulmonary edema. Positive-pressure ventilation with the addition of positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) may not reduce lung water but may increase access to previously collapsed regions of the lung and may increase the capacity of the interstitium to hold fluid.
Peritoneal fluid
A large volume of fluid in the abdomen can increase intraabdominal pressure (so-called abdominal compartment syndrome) and should be drained before anesthesia if feasible. Abdominal compartment syndrome is associated with a number of physiologic changes, including hypoventilation with reduced pulmonary compliance; tachycardia; low cardiac output; and increased central venous pressure (CVP), mean pulmonary artery pressure, and PCWP. In the abdomen, the increased pressure reduces urine output and decreases blood flow to the abdominal wall and the splanchnic vascular beds. Intraabdominal hypertension also may increase intracranial pressure (ICP) with a decrease in cerebral perfusion pressure.94
Drainage of the abdomen usually is achieved by placing a catheter in the abdominal cavity and drawing off the fluid with a syringe. It is helpful if the catheter has additional side holes cut in it before insertion so that there is less likelihood of the catheter being obstructed by the omentum. Most affected animals have greater respiratory distress lying on their backs, and the catheter usually is inserted with the animal on its side. I usually place the catheter about halfway between the last rib and the ischium, 1 to 4 inches off the ventral midline. Draining fluid in this manner can take a long time, but this is actually advantageous because rapid removal can result in mesenteric vasodilatation and cardiovascular collapse.94 In the case of hemoabdomen, the blood may have been defibrinated, but it is best to collect it in an anticoagulant (e.g., heparin, citrate). Collected blood should be used only if there is no gross contamination of the abdomen and no risk of neoplasia. The blood should be passed through a filter to remove clots before it is autotransfused. In cases of massive trauma, it may be better to leave the blood in the abdomen until the surgeon is ready to stop the bleeding. Although the accumulated blood may compromise ventilation during this time, the increased intraabdominal pressure may reduce the rate of hemorrhage.
Increased intracranial pressure
Increased ICP requires careful management in terms of fluid balance. The cranial vault is a relatively fixed cavity, and any accumulated fluid tends to increase the pressure. An increase in the fluid content of the brain or in the volume of blood or cerebrospinal fluid in the cranial vault promotes an increase in ICP. In situations in which the cause is medically reversible (e.g., hyponatremia), therapy should be carried out before anesthesia. In cases in which the diagnosis or treatment requires anesthesia, the preoperative assessment of the patient must include a detailed examination of fluid balance. Animals with an acute increase in ICP caused by trauma also may be hypovolemic because of other injuries. Judicious use of hypertonic resuscitation fluids is appropriate for these patients because such fluids promote a reduction in ICP while restoring circulating volume.103,199 Patients with chronically increased ICP often have had decreased food and water intake for some time and may have been treated with diuretics to reduce ICP. Consequently, such patients often are dehydrated and may have electrolyte disturbances. Whenever possible, preoperative assessment should include examination of the animal for signs of dehydration, an assessment of the cause of increased ICP, an evaluation of renal function, and measurement of serum electrolytes, hematocrit, total proteins, osmolality, and colloid osmotic pressure. If the animal clearly is dehydrated, it should be given fluids before anesthesia to increase its circulating volume. If plasma osmolality is less than 320 mOsm/kg, it may be beneficial to treat the animal with mannitol (0.25 to 1 g/kg).
Pregnancy
Pregnancy is associated with many changes in fluid balance. In women, the typical changes associated with pregnancy include hyponatremia; decreased blood urea nitrogen and creatinine concentrations; respiratory alkalosis; decreased serum calcium, magnesium, and protein concentrations; and decreased hematocrit. Similar changes have been documented in dogs. Serum protein concentrations tend to decrease during pregnancy, with the most marked change being a decrease in serum albumin concentration.28 Hematocrit decreases with a proportionately greater decrease with increasing numbers of fetuses.5,99 The pregnant dog has a decreased baroreceptor response to hypotension and is more susceptible to hypotension with blood loss.22,23 Thus the pregnant animal may be more susceptible to the negative circulatory effects of anesthetics and may require an increased volume of fluids during a surgical procedure. In bitches and queens that have been in labor for some time, dehydration and endotoxemia also may be present and add to circulatory instability. Affected patients may benefit from fluid therapy before anesthesia.
Changes in function
Cardiovascular disease
If the heart is failing, it may not tolerate an increased fluid load. Increased preload in this setting may not result in increased cardiac output because of changes in the Frank-Starling curve. Conversely, even a failing heart does not function optimally if preload is allowed to decrease too much. In a prospective study of human patients, it was found that the frequency of postoperative heart failure was highest in patients who had received less than 500 mL/hr of fluids intraoperatively.34 The most common cause of congestive heart failure in dogs is mitral insufficiency. This condition is characterized by excessive retrograde flow with an increasing volume load on the heart. Treatment often involves use of vasodilators (e.g., nitroglycerin, hydralazine, angiotensin-converting enzyme inhibitors) to decrease afterload, and diuretics and salt restriction to decrease circulating volume. Consequently, cardiac patients have the potential to be hypovolemic. The diagnosis of relative hypovolemia in these patients is based on clinical signs, such as skin turgor, mucous membrane color, capillary refill time (CRT), and jugular venous distention. Evaluation of renal function (including urine output) may assist in deciding whether the animal is adequately hydrated. Thoracic radiographs can be used to help assess pulmonary venous distention (i.e., lack of pulmonary venous distention implies lower left atrial pressure and hence a lack of excessive preload). The most useful measurement in these patients is PCWP. PCWP is obtained by inserting a balloon-tipped catheter into the pulmonary vein from either the jugular or femoral vein. Such invasive monitoring certainly is warranted in some cardiac patients and provides the best guide to fluid therapy. If the animal has right-sided heart failure, monitoring CVP provides similar information. In one study, use of CVP or PCWP was associated with more aggressive fluid therapy (>500 mL/hr), which in turn was associated with a lower risk of postoperative congestive heart failure.34 In the past, it has been recommended that fluids containing low concentrations of sodium be administered to cardiac patients (e.g., 0.45% saline in 2.5% dextrose). Most of these patients have an increase in total body sodium and an increase in total body water. The latter tends to exceed the former, and affected patients may be hyponatremic.7 Thus it seems illogical to give a solution that contains additional free water. If such a patient is hypovolemic, it is more appropriate to use a balanced electrolyte solution. If the patient is not hypovolemic or it already has excessive volume, fluids may not be needed.
Coagulation defects
Any coagulation defect that is likely to increase intraoperative blood loss should be corrected before surgery if possible. If an animal has a known coagulation defect (e.g., hemophilia, hepatic failure, coumarin poisoning, von Willebrand’s disease), it should be given the appropriate therapy such as fresh frozen plasma, cryoprecipitate, fresh plasma, or fresh whole blood, and vitamin K in the case of coumarin poisoning. These treatments should be given within a few hours of surgery because the half-lives of most clotting factors are relatively short. Although fresh frozen plasma and fresh plasma may have sufficient clotting factors to reverse the coagulation defect, such therapy often fails in animals with severe defects. In dogs with von Willebrand’s disease, infusion of cryoprecipitate is a more effective treatment than fresh frozen plasma alone.35,175 Therapy with plasma from donors receiving desmopressin (DDAVP) may be more effective than plasma from untreated donors.96 When DDAVP is given to dogs with typ. 1 von Willebrand’s disease, there is a measurable increase in the binding of von Willebrand factor to collagen, suggesting an improvement in clotting ability during surgery.97 Cryoprecipitate often is prepared from a number of donors and therefore has the potential to provide greater antigenic stimulation or transmit disease. Cryoprecipitate contains 10 to 20 times the normal amount of clotting factors and can be given in a small volume. Thus it may be useful in animals in which volume overload may be a concern (e.g., Doberman pinschers with von Willebrand’s disease and cardiomyopathy). I have used cryoprecipitate but also have successfully managed dogs with von Willebrand’s disease using fresh frozen plasma in mildly affected dogs or by treating both the plasma donor and recipient with DDAVP (1 μg/kg subcutaneously) in more severely affected dogs. Recommendations for the dosage of fresh frozen plasma range from 6 to 30 mL/kg and for cryoprecipitate from 1 U/5 to 15 kg.175 DDAVP also may be useful in restoring platelet function in some cases of iatrogenic platelet dysfunction. It has been used to treat increased bleeding times associated with aspirin administration and also platelet defects associated with cardiopulmonary bypass.138,156,193
Note: Volumes must be expressed in the same units (i.e., blood volume in microliters if platelet count is per microliter or platelet count per liter if blood volume is in liters). The dosage of a platelet-rich plasma could be determined using a similar approach but there is uncertainty about the actual dosage required.168
The ASA guidelines for infusion of platelets are as follows:143
1. Prophylactic platelet transfusion is rarely indicated when thrombocytopenia is caused by increased platelet destruction (e.g., idiopathic thrombocytopenic purpura).
2. Prophylactic platelet transfusion is rarely indicated when thrombocytopenia is caused by decreased platelet production when the platelet count is greater than 100 × 109/L and is usually indicated when the platelet count is less than 50 × 109/L. The determination of whether patients with intermediate platelet counts (50 to 100 × 109/L) require therapy should be based on the risk of bleeding.
3. Surgical and obstetric patients with microvascular bleeding usually require platelet transfusion if the platelet count is less than 50 × 109/L and rarely require therapy if it is greater than 100 × 109/L. With intermediate platelet counts (50 to 100 × 109/L), the determination should be based on the patient’s risk for more significant bleeding.
4. Operative procedures ordinarily associated with insignificant blood loss may be undertaken in patients with platelet counts less than 50 × 109/L.
5. Platelet transfusion may be indicated despite an apparently adequate platelet count if a known platelet dysfunction and microvascular bleeding are present.
More recent guidelines also suggest that platelet numbers should not be allowed to decrease to less than 50 × 109/L during massive transfusion and should be greater than 100×109/L in patients with multiple trauma or central nervous system injury.77 A platelet count as low as 5 x 109/L is an effective transfusion trigger in human patients with thrombocytopenia who are not undergoing invasive procedures.168
Endocrine disease
Diabetes mellitus
In controlled diabetes, there rarely is any major concern about fluid balance preoperatively. The animal’s normal feeding regimen and insulin dose are used on the day before surgery. On the morning of surgery, the animal receives one third to one half of its daily dose of insulin, and blood glucose concentration is monitored throughout the procedure.106 The animal is treated with glucose, insulin, or some combination of these as determined by serial blood glucose measurements. Animals with uncontrolled diabetes may be dehydrated and may require fluid therapy before anesthesia.
Access to the circulation
The technical aspects of fluid administration are covered in Chapter 15. In the perioperative period, access to the circulation via the intravenous or intraosseous route should be available so that fluids can be given rapidly should the need arise. As discussed earlier, the diameter of the catheter should be sufficient to allow fluids to be administered rapidly enough for the expected deficits. It also is important that the connections to the animal be set up carefully and that they are secure. If the fluid line becomes disconnected with the animal draped for surgery, it may not be detected quickly, and the animal may experience substantial blood loss from the catheter. When the patient is prepared for a surgical procedure, the anesthetist should make sure to set up the fluid lines so that an injection port is accessible without the need to reach under the drapes. The animal also should be positioned in such a way that the fluids can flow easily. Drugs added to the fluids and administered through the same line must be compatible (see Table 17-1). If an animal will be receiving several drugs, it may be necessary to create additional access sites to prevent incompatible drugs from being administered through the same line. Consideration also must be given to the site of access. In cats undergoing declawing of the front paws, it is advisable to place the catheter in the hind leg so that it does not interfere with the surgery. When the caudal vena cava is to be occluded during surgery, it is important to have the catheter in the forelimb or neck so that fluids reach the remaining circulation during the occlusion. In an emergency in an anesthetized animal with no venous access, the most visible vessel usually is the sublingual vein. This vein can be catheterized rapidly if necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree