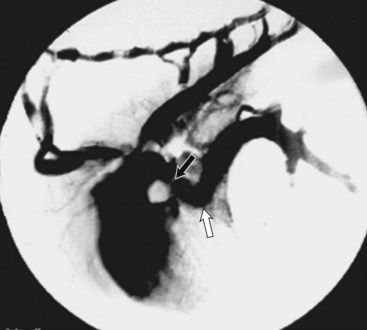
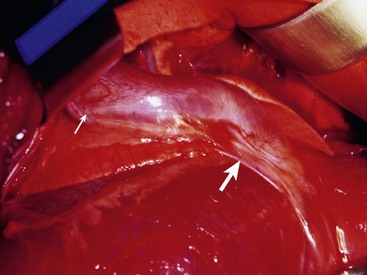
Chapter 107 The pericardium is a saclike structure that envelopes the heart; root of the aorta and pulmonary artery; and termination of the vena cavae, pulmonary veins, and azygos vein. It consists of two layers: the outer, fibrous pericardium and inner, serous pericardium.30 Fibrous pericardium is a thin, tough tissue covered on its outer surface by the mediastinal pleura. The base of the fibrous pericardium blends with adventitia of the great arteries and veins of the heart. Serous pericardium forms a closed, mesothelial-lined cavity and consists of parietal and visceral layers. The visceral layer of serous pericardium is the portion that invaginates over the heart, adhering tightly to become the smooth covering known as the epicardium. The epicardium is attached firmly to the myocardium except along the grooves containing coronary vessels and fat. The uninvaginated, parietal layer of serous pericardium forms the remainder of the serous layer and is firmly fused to the fibrous pericardium. Unlike the parietal serous pericardium, which is composed of interlacing collagenous fibers, the stromal layer of the visceral pericardium consists of elastic fibers.30 The fibrous pericardium inserts on the aortic arch and brachiocephalic trunk, the left atrium at the level of the pulmonary veins, and the right heart dorsal to the interatrial groove. The caudoventral apex of fibrous pericardium anchors ventrally at the muscular insertion of the diaphragm (sternopericardiac ligament).30 The pericardium is supplied by paired pericardial branches of the internal thoracic arteries, which course caudoventrally across the ventrolateral aspect of the pericardium. The pericardiacophrenic arteries, which also arise from the internal thoracic arteries, course with the phrenic nerves and supply the dorsolateral aspects of the pericardium. The pericardium has parasympathetic and sympathetic innervation, with sympathetic fibers carried by the ventrolateral and ventromedial cervical cardiac nerves.5,30,64 In regards to the pericardium, Shabetai writes, “The major function of most organs is readily apparent and requires no deep knowledge of biology or physiology. However, whether or not the pericardium subserves an important function or not has been debated over the years, and the debate continues.”62 The pericardium provides a gliding surface to accommodate heart motion. The pericardial cavity is filled with a variable amount of pericardial fluid; in dogs, fluid volume ranges from 1 to 15 mL. Pericardial fluid is an ultrafiltrate of serum that has phospholipids for lubrication, a protein content of 1.7 to 3.5 g/dL, and colloid osmotic pressure approximately 25% of that seen in serum.40,61 Because the pericardium is noncompliant and has a small reserve volume, intrapericardial pressure rises rapidly when the volume of its contents increases acutely. Chronic stretching of the pericardium results in hypertrophy and augmentation of the pericardial volume. Capacitance of the pericardium is influenced by rate of fluid accumulation. Because parietal pericardium is fairly noncompliant, pericardial pressure begins to increase after 5 to 60 mL of fluid accumulates acutely within the pericardial sac. With slow accumulation, the pericardium stretches, permitting augmentation of pericardial volume and rightward shifting of the pressure-volume curve. As a result, the pericardium can accumulate a larger volume of fluid before pressure begins to increase. However, beyond a certain point, pressure increases quickly with small increases in volume. When the pericardium is thickened, as is the case with constrictive pericardial disease, a minor increase in volume causes a significant increase in pericardial pressure.* An increase in pericardial pressure increases diastolic pressure within the heart, which in turn reduces stroke volume. Pericardial pressure first equilibrates with right ventricular filling pressure (right-sided heart tamponade) and then with left ventricular filling pressure (left-sided heart tamponade). With tamponade, cardiac output decreases, and systemic venous pressure increases, stimulating activation of compensatory neurohumoral responses to increase vascular volume and maintain blood pressure.† With activation of the renin-angiotensin-aldosterone system, sodium and water are retained. Sympathetic stimulation and adrenomedullary catecholamine release produce positive inotropic and chronotropic effects and vasoconstriction. Because atrial wall stretching is limited by tamponade, atrial natriuretic peptide is not released with pericardial effusion and is therefore not available to counteract the effects of the renin-angiotensin-aldosterone system. As a result, cardiac tamponade is associated with increases in systemic venous and portal pressures, causing jugular distention, liver congestion, ascites, and peripheral edema secondary to fluid transudation from systemic capillary beds.† Although cardiac contractility is not directly affected by tamponade, compression of coronary arteries results in poor myocardial perfusion. When coupled with decreased cardiac output and arterial hypotension, cardiogenic shock and death may result. Arterial pressures may vary paradoxically with respiration during severe cardiac tamponade. During inspiration, pericardial pressure and right ventricular pressure decrease, facilitating venous return to the right atrium and ventricle and pulmonary blood flow. However, because heart volume is limited by the pericardium, the intraventricular septum shifts to the left. Consequently, left ventricular end-diastolic volume, left heart output, and arterial pressure are decreased during inspiration, resulting in variation of systolic arterial pressures often greater than 10 mm Hg. This phenomenon, known as pulsus paradoxus, can also occur with obstructive lung disease, restrictive cardiomyopathy, or hypovolemic shock and is therefore not pathognomonic for cardiac tamponade.1,36,42,47,58,59,68,69 Absence of Pericardium and Pericardial Defects Absence of the pericardium is rare in dogs and cats. It does not precipitate clinical signs and is usually detected only at necropsy. Partial pericardial defects occur and represent a risk for cardiac herniation. Right atrial herniation through partial pericardial defects has been reported in dogs.33,37 Pericardial cysts are rare and have been described primarily in companion animals younger than 3 years of age. Cysts are either unilocular or multilocular masses. On histologic analysis, they are thought to be cystic hematomas because they do not have an epithelial lining cell. In some cases, cysts were associated with a peritoneopericardial diaphragmatic hernia. In other cases, cysts were on a stalk at the apex of the pericardium. This suggests that pericardial cysts result from entrapment of omentum, falciform ligament, or liver in the pericardium during development.48,51,63,65 If cardiac tamponade from excessive pericardial fluid is present, a pericardiocentesis or pericardial cystocentesis is required before surgery. Median sternotomy (see Chapter 104) facilitates exploration of the thoracic cavity, resection of the pericardial cyst, subtotal pericardiectomy, and repair of a peritoneopericardial diaphragmatic hernia if needed. The prognosis is good if the cyst can be removed completely. Rupture of the pericardium after trauma (e.g., automobile accident, blunt thoracic trauma) is rarely reported in dogs but likely occurs more frequently than diagnosed because it usually does not cause clinical signs.33 When the pericardium contracts around the herniated heart during healing, however, a stricture can develop that compresses the vena cavae. This can result in a Budd-Chiari syndrome with ascites and hepatomegaly, caval syndrome with swelling of the head and neck, or both. Thoracic radiographs are generally unremarkable, although the caudal vena cava may appear to be kinked cranial to the diaphragm. Peritoneal effusion and hepatomegaly may be seen on abdominal radiographs with caudal vena cava obstruction. Caval angiography is the most valuable technique for diagnosis of this condition (Figure 107-1). Contrast material is injected as a bolus proximal to the suspected constriction, and serial radiographs are taken to document the obstruction. Pressure measurement can be performed at the same time to demonstrate increased venous pressure upstream of the stenosis. Right-sided intercostal thoracotomy (see Chapter 104) at the fifth or sixth intercostal space provides exposure of the right atrium and vena cavae. Compression created by the pericardium should first be released by resecting the fibrotic pericardial sac (Figure 107-2). The pericardial sac may be displaced dorsal to the atrium, causing it to compress the caudal vena cava as it passes across its ventral aspect. Compression of the vena cava is released by resecting the fibrous band crossing the vena cava. If stricture of the wall of the vena cava is present, an angioplasty with an inlay pericardial patch graft can be performed. A Satinsky clamp is applied tangentially to the vena cava to isolate a segment of its wall. The vena cava is opened longitudinally, and an oval-shaped patch of autogenous pericardium is sutured to the edges of the incision with 5-0 polypropylene in a simple continuous pattern along each side of the patch. The clamp is released, and the site is examined for hemorrhage. Mild oozing will usually stop with digital pressure; if leakage at the patch site is of concern, additional interrupted sutures are placed. Pericardial effusions are categorized by characteristics of the accumulated fluid. A transudative pericardial effusion may occur with congestive heart failure, peritoneopericardial diaphragmatic hernia, hypoalbuminemia, or increased vascular permeability.6–8,12,15,23–25,49 An exudate (total protein >2.5 g/dL; total nucleated cell count >5000 cells/µL) results from infectious or noninfectious pericarditis, such as feline infectious peritonitis or feline cardiomyopathy.* Infectious agents can be bacterial, fungal, or viral. Fungal pericarditis is unusual, with the exception of Coccidioides immitis in dogs living in the southwestern United States.43,53 Bacterial pericardial effusion has been reported in dogs and is suspected to be secondary to grass awn migration.4 Causes of hemorrhagic pericardial effusion include trauma, neoplasia, anticoagulant intoxication, or rupture of the left atrium secondary to mitral valve disease.9,18,45,56,57,72 If the underlying condition cannot be determined, the cause is classified as idiopathic. Idiopathic pericardial effusion is considered by some authors to be the most common cause of acute or chronic hemorrhagic pericardial effusion in dogs.7,8,25,53,72 Some dogs with idiopathic pericardial effusion may actually have a small intrapericardial tumor or mesothelioma that is missed during diagnostics. In one study71 idiopathic pericardial effusion was originally diagnosed in 24 of 87 dogs; mesothelioma was subsequently confirmed in four of the dogs. Although infectious agents are an unlikely cause, influenza type A viral ribonucleic acid was detected in pericardial fluid from one of 14 dogs with idiopathic pericardial effusion.76 The second most common cause of hemorrhagic pericardial effusion is neoplasia of the heart, heart base, or pericardium, with hemangiosarcoma of the right atrium being the most common type of neoplasia.* Hemangiosarcoma is often multicentric, involving the spleen or liver at the time of pericardial effusion detection. Chemodectoma is the second most common cardiac tumor to cause pericardial effusion and is most often seen in brachycephalic dogs. Hemorrhagic pericardial effusion may also be caused by pericardial mesothelioma. This diffuse neoplasm of the pericardium and other serosal surfaces may be difficult to distinguish from idiopathic pericardial effusion, even with pericardial histopathology and immunohistochemistry.71 Dogs with pericardial effusion are often older (mean age, 9.7 ± 2.2 years) and large breed (mean weight, 31.2 ± 12.6 kg). Golden retrievers were overrepresented when compared with the general hospital population.3,71 Acute pericardial effusion causes acute hypotension, rapidly progressive weakness, dyspnea, collapse, and cardiogenic shock. The most common presentation for animals with left atrial tears, acute neoplastic hemorrhage, or traumatic laceration of a coronary artery is death.† Animals with chronic pericardial effusions present with a history of exercise intolerance, lethargy, anorexia, dyspnea, weakness, and gradual onset of abdominal distention. Occasionally, owners report vomiting or collapsing episodes. Manifestations of pericardial effusion depend on rates of effusion formation and increase in intrapericardial pressure. Classic findings for chronic pericardial effusion include muffled heart sounds, weak femoral pulses, tachycardia, and distention of jugular and peripheral veins. With cardiac tamponade, central venous pressure frequently exceeds 10 to 12 mm Hg. The lateral saphenous veins are usually distended and do not collapse when the animal is placed in lateral recumbency with the pelvic limb raised above the level of the heart. Chronic pericardial effusion commonly induces signs of right-sided congestive heart failure such as pleural effusion and abdominal distention from ascites or hepatomegaly.† Pulsus paradoxus, (i.e., variations of pressure quality associated with respiration phase) may be present in severe cases. Weight loss is common in dogs with chronic pericardial effusion.§ Although animals present with signs of chronic pericardial effusion more often than with acute pericardial effusion, animals with chronic pericardial effusion from idiopathic pericardial effusion or right atrial tumor may present with apparent acute manifestation because of acute bleeding onset. Findings may include evidence of cardiogenic shock (increased capillary refill time, pale mucous membranes, weak pulses, tachycardia). Clinical signs of the underlying disease, such as disseminated neoplasia or infection, may be present. A left apical systolic murmur suggests the possibility of a left atrial tear secondary to chronic mitral regurgitation.3,7,8,25,60 Cytology and fluid analysis are useful for classifying pericardial effusion as a transudate, exudate, or hemorrhage but usually do not differentiate the underlying cause.* Most dogs with pericardial effusion have sanguineous or serosanguineous, sterile, inflammatory exudates. The packed cell volume of the fluid is less than that of the peripheral blood. Because serosanguineous pericardial effusions are rapidly depleted of clotting factors, fluid samples will not clot in an activated clotting time tube unless active hemorrhage is present. Cytology of the pericardial fluid is not reliable for determining the presence of neoplasia.22,27,66 Reactive mesothelial cells are often present in animals with pericardial effusion and do not correlate with the presence of mesothelioma. If the cytology is suggestive of infection, a sample should be submitted for bacterial and fungal culture. Some authors have correlated results of effusate biochemical analysis with the presence of neoplasia. In one study, the pH of fluid was greater than 7.5 in effusates of neoplastic origin.27 In another study,22 pericardial fluid pH, bicarbonate, and chloride were significantly lower, and lactate, hematocrit, and urea nitrogen were significantly higher in dogs with neoplastic pericardial effusion compared with those of non-neoplastic origin. Because of the degree of overlap in the two groups, the clinical relevance of pericardial effusate analysis is limited.22,34
Pericardial Surgery
Anatomy
Function of the Pericardium
Pathophysiology of Cardiac Tamponade
Congenital Pericardial Disease
Pericardial Cysts
Treatment
Acquired Pericardial Diseases
Pathophysiology
Radiographic Findings
Surgical Treatment
Pericardial Effusion
Signalment and History
Physical Examination
Pericardial Fluid Cytology and Analysis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pericardial Surgery
Only gold members can continue reading. Log In or Register to continue