Chapter 60 Pancreas
Structure and Function
Structure
Macroscopic Anatomy
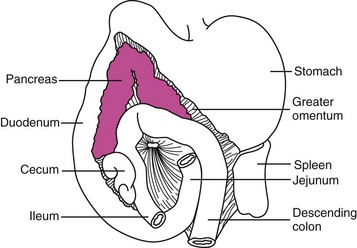
The pancreas is a bilobed structure in companion animal species. The right lobe lies in the mesoduodenum in close apposition to the proximal duodenum. The right lobe extends posteriorly from the pylorus to the cecum. The left lobe lies in the greater omentum and lies in close apposition to the transverse colon caudally and the stomach cranially (Figure 60-1). The dog typically has two pancreatic ducts: a ventral or accessory pancreatic duct and a dorsal pancreatic duct. The ventral duct is the larger of the two and drains the right pancreatic lobe, while the dorsal duct drains the left lobe. These ducts usually intercommunicate within the gland. The ventral pancreatic duct is sometimes absent in the cat. In the cat, the dorsal pancreatic duct merges with the common bile duct prior to entry into the proximal duodenum.1,2
Microscopic Anatomy
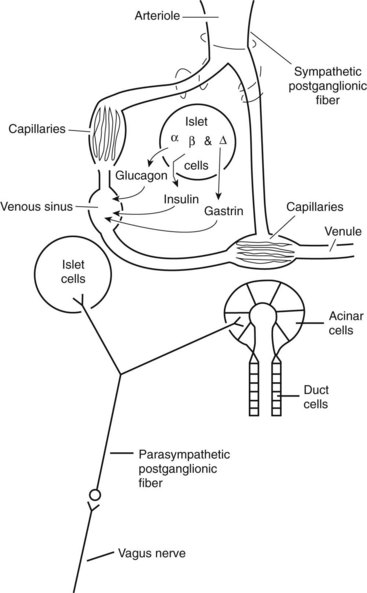
The exocrine pancreas is a tubuloalveolar gland with a division of function between the acinar cells, which secrete the digestive enzymes, and the duct cells, which add water, bicarbonate, chloride, intrinsic factor, and antibacterial proteins. Throughout the pancreatic parenchyma are isolated clusters of cells forming the islets of Langerhans (Figure 60-2). The islets contain four major types of endocrine cells that synthesize and secrete glucagon (A cell), insulin (B cell), somatostatin and gastrin (D cell), and pancreatic polypeptide (PP cell). Although these hormones have other well-known physiologic effects, they also have important endocrine or paracrine effects on the pancreatic acini because of the islet–acinar portal venous system. Insulin appears to have long-term effects on the regulation of the biosynthesis of pancreatic digestive enzymes and short-term effects on the potentiation of pancreatic secretory response to gut hormones and neurotransmitters. Other islet hormones and peptides, including glucagon, somatostatin, and pancreatic polypeptide, probably act as inhibitory regulators of the pancreatic acini.
Blood Supply
In the dog and cat the exocrine pancreas does not have a direct arterial blood supply. Instead, an islet–acinar portal blood system exists, that is, the acini are perfused by venous blood arising from the islet vasa efferentia. Because some blood courses first to the islets, which secrete hormones into the blood, and then to the acinar cells, which secrete enzymes into the juice in response to stimulation by hormones, the pancreas has the potential to autoregulate part of its own exocrine secretion (see Figure 60-2).
Innervation
The efferent nerve supply to the pancreas is both sympathetic and parasympathetic (see Figure 60-2). Sympathetic postganglionic fibers emanate from the celiac and cranial mesenteric plexuses and accompany the arteries to the organ. Parasympathetic preganglionic fibers are distributed by branches of the vagi coursing down the antroduodenal region. Vagal fibers terminate at either acini and islets or intrinsic cholinergic nerves of the pancreas. In general, the sympathetic nerves inhibit and the parasympathetic nerves stimulate pancreatic exocrine secretion.
Function
Duct Cells
Water, anions, and cations are secreted primarily by the pancreatic duct cells. Bicarbonate is necessary to neutralize gastric acid that is emptied into the small intestine during feeding to prevent damage to the intestinal mucosa. Bicarbonate secretion also provides an increase in duodenal pH that is necessary for optimal activity of the secreted enzymes, particularly lipases. A fluid secretion isotonic to plasma and high in bicarbonate concentration is stimulated by the endocrine hormone secretin and the neurotransmitter vasoactive intestinal polypeptide from the duct cells of the exocrine pancreas. The bicarbonate concentration increases with increasing flow rate, up to 150 mEq/L, while the chloride concentration correspondingly decreases, so that the sum of the anions remains constant. The cation concentrations are plasma-like and do not change with flow rate (Figure 60-3).
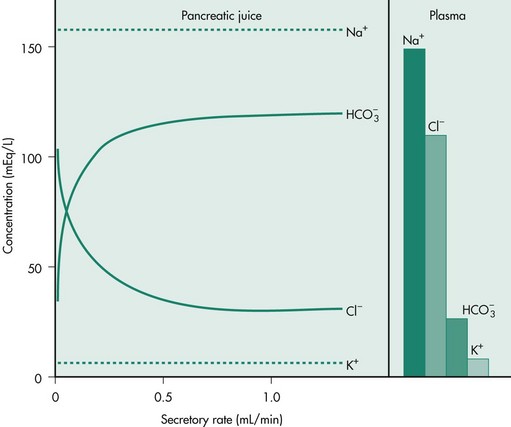
Figure 60-3 Chloride and bicarbonate secretion at low and high secretory flow rates from the canine pancreas.
(Reprinted with permission from Johnson LR: Gastrointestinal Physiology, 2nd ed, Philadelphia, Elsevier, 2007.)
Most mammals have developed a complex process for vitamin B12 (cobalamin) absorption involving secretion of a gastric intrinsic factor, binding of gastric intrinsic factor to cobalamin, and subsequent attachment of this intrinsic factor–cobalamin complex to specific receptors on ileal enterocytes. The dog and cat appear to have diverged from this pattern and instead rely mostly (dog) or exclusively (cat) on pancreatic intrinsic factor synthesis and secretion. Canine and feline pancreatic duct cells secrete a pancreatic intrinsic factor that is the primary mechanism for binding and receptor-mediated endocytosis of cobalamin in the gut.3,4
Acinar Cells
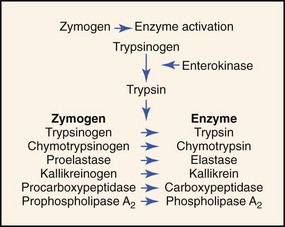
The pancreatic acinar cell secretes its proteolytic enzymes in precursor form (zymogens). Other enzymes (amylase, lipase, ribonuclease) are secreted in an active form. The enzymes in pancreatic fluid have the ability to hydrolyze dietary starch (amylase), fats (lipase), proteins (trypsin, chymotrypsin, carboxypeptidase, elastase), and nucleic acids (ribonuclease, deoxyribonuclease; Box 60-1). The action of enterokinase, an enzyme secreted by the duodenal mucosa, activates trypsinogen into proteolytically active trypsin. Trypsin then acts autocatalytically to activate trypsinogen and other proteolytic zymogens (Figure 60-4).
Box 60-1
Enzymes Secreted from Acinar Cells in the Exocrine Pancreas
Endopeptidases: hydrolyze interior peptide bonds of polypeptides and proteins.
• Trypsin—attacks peptide bonds involving basic amino acids.
• Chymotrypsin—attacks peptide bonds involving aromatic amino acids, leucine, and glutamine.
• Elastase—attacks peptide bonds involving neutral and aliphatic amino acids.
Exopeptidases: hydrolyze external peptide bonds.
• Carboxypeptidase A—active against peptides with aromatic and aliphatic amino acids at the C-terminus.
• Carboxypeptidase B—active against peptides with basic amino acids at the C-terminus.
• Amylase—hydrolyzes dietary starch into the disaccharide maltose and α-limit dextrins.
• Lipase—hydrolyzes triglycerides into free fatty acids, 2-monoglycerides, and glycerol.
• Phospholipase—hydrolyzes phospholipids to 1-lysophospholipids and free fatty acids.
• Ribonuclease—releases pyrimidine nucleotides from polyribonucleotides.
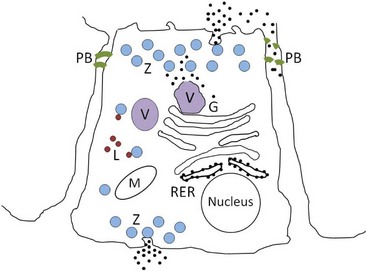
Pancreatic acinar cells protect themselves from intraacinar activation of zymogen and acinar cell necrosis through several mechanisms: (a) potentially harmful digestive enzymes are synthesized in the form of inactive precursors or zymogens in the rough endoplasmic reticulum; (b) zymogens are then transported to the Golgi complex where they undergo selective glycosylation. Lysosomal hydrolases that are eventually packaged in lysosomes are separated from zymogens bound for export as they pass through the Golgi complex. Lysosomal hydrolases are first phosphorylated at the six position of mannose residues, bound to receptors specific for 6-phosphoryl mannose, and then transported to lysosomes where the acid pH favors their dissociation from the receptors. Digestive enzymes lack the 6-phosphoryl mannose label, and are instead transported vectorially into a different secretory fraction; (c) packaging of zymogens into maturing zymogen granules sequesters them from contact with other subcellular fractions; (d) pancreatic secretory trypsin inhibitor (PSTI) is incorporated into the maturing zymogen granules. PSTI inactivates trypsin should there be any intraacinar activation of trypsinogen; (e) following stimulation (e.g., feeding and cholecystokinin secretion), mature zymogen granules and their contents are released from the cell into the ductal lumen in a process of membrane fusion and exocytosis; and (f) finally, zymogens are activated physiologically only after they enter the duodenum, where the brush-border enzyme enteropeptidase activates trypsinogen, and trypsin then activates other pancreatic zymogens (Figure 60-5).
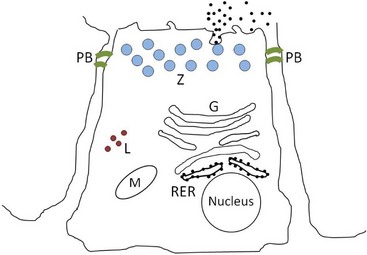
Figure 60-5 Normal pancreatic acinar cell.
(Courtesy of Drs. Panagiotis Xenoulis and Joerg Steiner, Texas A & M University, College Station, Texas.)
A large body of experimental, and some clinical, evidence suggests that the initiating event of acute pancreatitis is the premature activation of digestive zymogens within the acinar cell.5–8 Premature activation of digestive zymogen results in acinar cell necrosis and pancreatic autodigestion. In acute pancreatic necrosis, protein synthesis and intracellular transport to the Golgi complex appear to be normal, but digestive zymogens then become colocalized along with lysosomal hydrolases in large vacuoles. Cell biology studies reveal that lysosomal and zymogen granule fractions become colocalized through a process known as crinophagy, a process used by many cells to degrade accumulated secretory products when the need for secretion is no longer present. Although this process takes place in other cells without adverse consequences, it can be lethal in pancreatic acinar cells because of the peculiarity of their secretion products (digestive zymogens). Lysosomal hydrolases, such as cathepsin B and N-acetyl glucosaminidase, activate trypsinogen to the active trypsin form, and the enhanced fragility of these large vacuoles permits release of active enzyme into the cell cytoplasm (Figure 60-6). Trypsin acts autocatalytically to activate other trypsinogen molecules and other zymogens, each inducing a unique chemical pathology in pancreatic and extrapancreatic cells. A variety of inflammatory mediators and cytokines, interleukins, nitric oxide, and free radicals are involved in the further evolution of pancreatic acinar cell necrosis and inflammation, and often determine the outcome.9 Thus, a bout of pancreatitis begins with an initiating event (e.g., ischemia, inflammation, or ductal obstruction) followed by acinar events (e.g., colocalization, enzyme activation, and cell injury), the outcome of which is influenced by severity determinants (e.g., inflammatory cytokines, reactive oxygen species, altered oxidation-reduction state, and apoptosis).9 The further evolution of acute pancreatic necrosis to a systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome is determined by the balance of proinflammatory and antiinflammatory cytokines.9
Regulation of Secretion
Exocrine pancreatic secretions are regulated by hormonal, neural, and paracrine input during the cephalic, gastric, and intestinal phases of secretion. In the cephalic phase of exocrine pancreatic secretion, acetylcholine released by vagal postganglionic neurons stimulates H+ ion secretion by parietal cells (Figure 60-7). Gastric acid evokes duodenal secretin release, which then stimulates pancreatic fluid and bicarbonate secretion. Vagal stimulation also releases gastrin from antral G cells. In the dog, gastrin is equipotent with cholecystokinin (CCK) in stimulating pancreatic enzyme secretion. Gastrin stimulates the parietal cells to secrete H+.
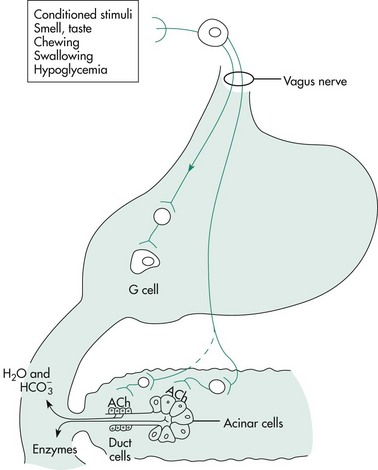
Figure 60-7 The cephalic phase of exocrine pancreatic secretion.
(Reprinted with permission from Johnson LR: Gastrointestinal Physiology, 2nd ed, Philadelphia, Elsevier, 2007.)
In the gastric phase of exocrine pancreatic secretion, the same essential mechanisms are involved as those in the cephalic phase of pancreatic secretion (Figure 60-8). Protein digestion products in the stomach release gastrin, resulting in the stimulation of pancreatic enzyme secretion and gastric acid secretion. Gastric distention stimulates gastric mechanoreceptors, which in turn stimulate parietal cells through vagal reflexes. H+ stimulates duodenal secretin release.
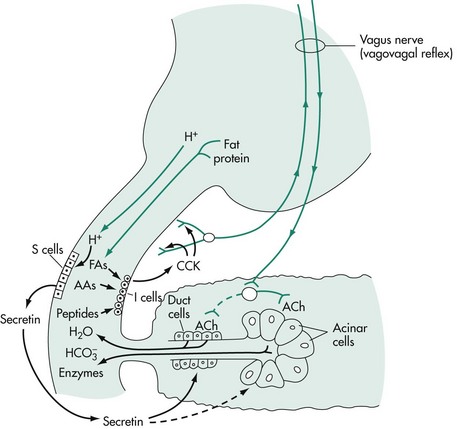
Figure 60-8 The gastric and intestinal phases of exocrine pancreatic secretion.
(Reprinted with permission from Johnson LR: Gastrointestinal Physiology, 2nd ed, Philadelphia, Elsevier, 2007.)
The intestinal phase of exocrine pancreatic secretion is the major phase of secretion (see Figure 60-8). The stimulus for the alkaline component from the duct cells is the hormone secretin. The only potent releaser of secretin is hydrogen ion. CCK is the principal humoral stimulant of enzyme secretion from the pancreatic acinar cells and is released physiologically in response to amino acids and fatty acids in the small intestine.
Diagnostic Evaluation of the Pancreas
Panagiotis G. Xenoulis, Jörg M. Steiner
Exocrine pancreatic disorders are common in clinical practice and pancreatitis is by far the most common disorder of the exocrine pancreas in both dogs and cats. Clinical diagnosis of pancreatitis can be challenging and it has been suggested that most cases of canine and feline pancreatitis remain undiagnosed. This is supported by necropsy studies in both dogs and cats that report that histopathologic evidence of pancreatic inflammation is common in both species, even in patients that are not clinical.1–3 Pancreatitis may be accompanied by relatively uncommon pancreatic complications such as pancreatic abscesses and pancreatic pseudocysts.
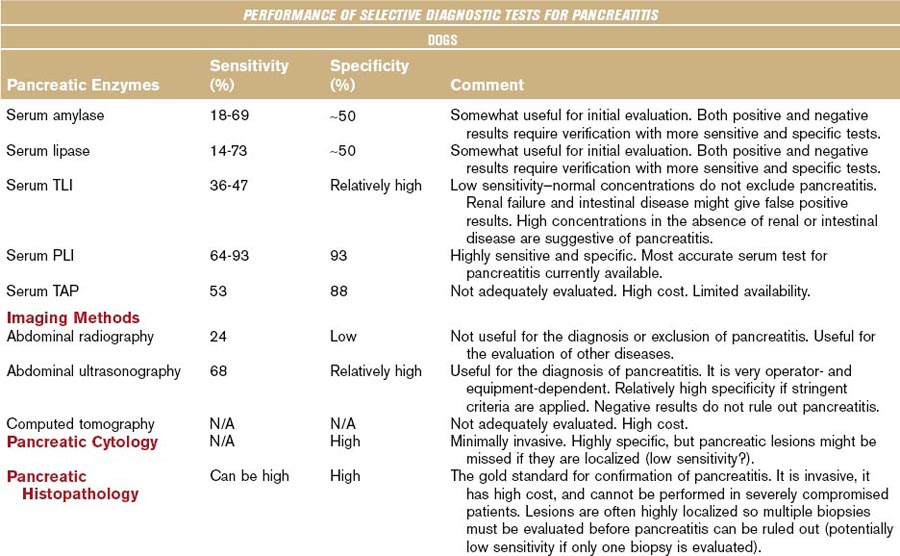
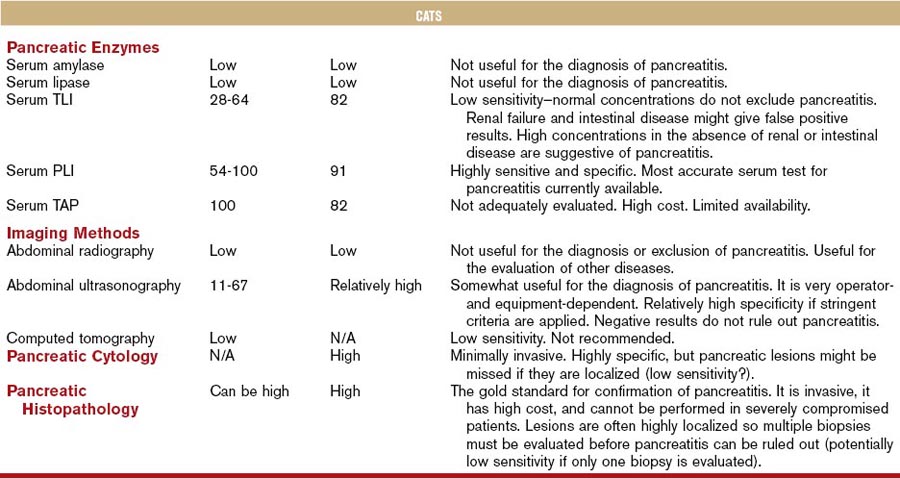
Although the diagnostic evaluation of any patient, including those with exocrine pancreatic disease, should always take into consideration the clinical presentation and general clinicopathologic findings, this chapter mainly focuses on the diagnostic evaluation of the pancreas using diagnostic modalities that are believed to specifically assess pancreatic structure, function, and/or pathology. Table 60-1 summarizes the clinical performance of commonly used diagnostic modalities with regards to the diagnosis of pancreatitis in dogs and cats.
Table 60-1 Biochemical and Imaging Findings in Dogs and Cats Affected with Acute Necrotizing Pancreatitis
Pancreatitis
Signalment, History, and Risk Factors
Although dogs of any age, breed, or sex can develop pancreatitis, certain groups might be predisposed. Most dogs presented with pancreatitis are middle-aged or older (usually more than 5 years old).4,5 Several breeds have been reported or suspected to be at increased risk (e.g., Miniature Schnauzers, Yorkshire Terriers, Cocker Spaniels, Cavalier King Charles Spaniels, Collies, and Boxers) but none of these predispositions are consistent among studies.3–6 Also, no clear sex predisposition has been identified.
Several pathologic conditions have been identified as potential risk factors for pancreatitis in dogs and, although a cause-and-effect relationship has not been established for most of them, their presence along with compatible clinical signs may raise the concern for pancreatitis. Many dogs with pancreatitis are overweight or obese.5 Also, endocrinopathies such as hyperadrenocorticism, hypothyroidism, and diabetes mellitus may be risk factors for pancreatitis.5 A history of drug administration (e.g., potassium bromide, phenobarbital, azathioprine, L-asparaginase, meglumine antimonite) in conjunction with compatible findings should also raise a concern for pancreatitis.5,7 Hypertriglyceridemia, when severe (higher than approximately 850 mg/dL), is a risk factor for pancreatitis in Miniature Schnauzers.8 This might also be true for dogs of other breeds that exhibit severe hypertriglyceridemia, but this has not yet been proven. Dietary factors (e.g., getting into the trash, consuming table scraps, ingestion of “unusual” food) and surgery at any time prior to diagnosis of pancreatitis also have been suggested as risk factors for pancreatitis in dogs.6
Similarly to dogs, cats of any age, breed, or sex can develop pancreatitis. Older cats appear to be more likely to develop chronic pancreatitis.2,9–11 Domestic shorthair and Siamese breeds are reported to be at an increased risk in some studies, but this has not been confirmed by other studies.2,9–11
Clinical Signs and Physical Examination Findings
Dogs with pancreatitis can present with a wide variety of clinical signs, which can range from mild partial anorexia with no apparent gastrointestinal signs to cardiovascular shock, disseminated intravascular coagulation (DIC), and death. There is no single clinical sign or combination of clinical signs that is pathognomonic for pancreatitis in dogs. Recent reports suggest that pancreatitis may be subclinical in some cases, or be associated with only mild and nonspecific clinical signs such as anorexia and weakness.1 In more typical cases, in addition to anorexia and weakness, dogs present with vomiting, diarrhea, and/or abdominal pain.5,12 The concurrent signs of vomiting and cranial abdominal pain is considered suggestive, but not pathognomonic, of pancreatitis in dogs. Evidence of dehydration, abdominal pain, icterus, fever or hypothermia, icterus, bleeding diathesis, or ascites may be seen on physical examination.5 Severe systemic complications (e.g., cardiovascular shock, DIC, or multiorgan failure) might occur in patients with severe pancreatitis.12,13 Other clinical signs may be observed as a consequence of concurrent disease (e.g., polyuria/polydipsia in animals with diabetes mellitus).5
The most common clinical signs in cats with pancreatitis do not specifically indicate gastrointestinal disease and include complete or partial anorexia and lethargy.9–1114 Vomiting, weight loss, and diarrhea are reported less commonly in the cat.9–1114 Abdominal pain may be evident in cats with acute pancreatitis but could be missed during routine physical examination. The most common physical examination findings are dehydration, pallor, and icterus.9–1114 Tachypnea and/or dyspnea, hypothermia/fever, tachycardia, signs of abdominal pain, and a palpable abdominal mass may also be noted.9–1114 Severe systemic complications (e.g., DIC, pulmonary thromboembolism, cardiovascular shock, and multiorgan failure) occasionally may be seen in cats with severe pancreatitis.
Routine Clinical Pathology5,9–12,14,15
Clinical Enzymology
Serum Pancreatic Lipase Immunoreactivity Concentration
There are multiple circulating lipases of various cellular origins (e.g., pancreatic, hepatic, and gastric) and all of them share the same function (i.e., hydrolysis of triglycerides). Therefore, in assays of lipase activity, many of the different lipases may contribute to the total serum lipase activity. More recently, it was shown that lipases of different cellular origins are encoded by different genes and consequently have differing amino acid sequences. Pancreatic lipase is expressed exclusively by pancreatic acinar cells and is structurally different from other lipases. Thus immunoassays for the specific measurement of pancreatic lipase have been developed and analytically validated for dogs and cats.16,17 In contrast to the traditional activity assays for lipase, which indiscriminately measure the activity of lipases of any origin, these immunoassays specifically quantify lipases based on their unique structure. Consequently, they are considerably more useful for exocrine pancreatic disease than assays for serum lipase activity. During pancreatitis pancreatic lipase leaks from acinar cells and enters the circulation in larger than normal quantities and can be detected by specific immunoassays for pancreatic lipase.
The originally developed immunoassays for pancreatic lipase were in-house immunoassays that used polyclonal antibodies and had limited availability. Commercial immunoassays (e.g., Spec cPL for dogs and Spec fPL for cats) are now more routinely available.18,19
Canine pancreatic lipase is believed to be exclusively of pancreatic origin.20–22 An immunolocalization study has identified the pancreatic acinar cell as the cell of origin.20 Dogs with EPI have near total absence of serum canine pancreatic lipase immunoreactivity (cPLI) again suggesting that cPLI is likely of exocrine pancreatic origin.21 The specificity of Spec cPL was reported to be 96.8% in another study of 31 dogs with normal pancreatic histology.22 In a recent multicenter study, the specificity of cPLI was estimated to be at least 78% in dogs with a clinical diagnosis of pancreatitis.23 Experimentally induced chronic renal failure and prednisone administration have not been found to have any clinically significant effect on serum cPLI concentration.24,25
Serum cPLI concentration is also sensitive for the diagnosis of pancreatitis in dogs.18,23,26,27 The reported sensitivity of cPLI for the diagnosis of canine pancreatitis ranges from 64% to 93%, depending on the severity of the disease. This is considerably higher than the sensitivity reported for serum canine trypsin-like immunoreactivity (cTLI) concentration (36.4% to 46.7%), serum amylase activity (18.2% to 73.3%), and serum lipase activity (13.6% to 69%), and is similar to or higher than that of abdominal ultrasound (67% to 68%) performed by a board-certified radiologist.5,18,23,26–28 In a recent preliminary report of a multicenter study, the sensitivity of this assay was estimated at 93%.23 Because of its high sensitivity, normal serum cPLI concentrations make a diagnosis of clinically relevant pancreatitis very unlikely. However, it remains to be determined if, as a consequence of its high sensitivity, cPLI detects pancreatic pathology that is not clinically relevant. Based on the aforementioned studies, serum cPLI concentration is the most sensitive and specific test currently available for pancreatitis in dogs.
Studies in cats with both experimental and spontaneous pancreatitis have shown that serum feline pancreatic lipase immunoreactivity (fPLI) concentration is very sensitive for pancreatitis.29–31 In one of these studies, fPLI was found to be 100% sensitive for moderate to severe spontaneous feline pancreatitis, which was superior to the sensitivities of serum feline trypsin-like immunoreactivity (fTLI) concentration (28%) or abdominal ultrasound (80%).29 In a recent preliminary report, the sensitivity of serum fPLI concentration was reported at 78%.31 Considering the overall sensitivities for pancreatitis reported for serum fPLI concentration (67% to 78%), fTLI (28% to 64%), and abdominal ultrasonography (11% to 67%), serum fPLI concentration currently appears to be the most sensitive test for the diagnosis of feline pancreatitis.29,31–35 The specificity of serum fPLI concentration of 82% to 91%, has been reported to be superior to that of fTLI (82%) or abdominal ultrasound (73%).15,29,31 Further studies are needed to confirm the reproducibility of these findings, but serum fPLI concentration currently appears to be the most useful test for the diagnosis of feline pancreatitis.
Serum Amylase and Lipase Activities
Serum amylase and lipase activity assays have long been considered markers for pancreatitis in dogs.36,37 Serum activities of these two enzymes increase during experimental canine pancreatitis, but studies of spontaneous canine pancreatitis have shown poor sensitivity and specificity.18,36–41 Gastric mucosal and hepatic parenchymal amylases and lipases are routinely detected in activity assays, which has contributed to limitations in sensitivity and specificity. Moreover EPI and pancreatectomized dogs both have significant residual circulating lipase and amylase activity, indicating that tissues other than the pancreas account for a large portion of the serum activity of lipase and amylase.21,41 Traditional catalytic assays, are not able to differentiate amylases and lipases according to their tissue of origin. This leads to a low specificity of serum amylase and lipase activities for pancreatitis in dogs.28,36
Many dogs that have extrapancreatic disease have increased serum lipase and/or amylase activities.36 The main nonpancreatic conditions associated with increased serum amylase and/or lipase activities include renal, hepatic, intestinal, and neoplastic diseases, as well as corticosteroid administration (only for lipase activity). It has been suggested that only increases of amylase and lipase activities of more than three to five times the upper limit of the reference range should be considered suggestive of pancreatitis in dogs, so as to increase the specificity of these assays.42,43 However it has been shown that even such increases can result from nonpancreatic disorders.28,36,43,44 Therefore increased serum amylase and/or lipase activities do not confirm the presence of pancreatitis in any case and more specific tests need to be utilized.
The sensitivity of serum amylase and lipase activities for spontaneous canine pancreatitis varies but is generally low (32% to 73% for lipase activity and 41% to 69% for amylase activity) and it is even lower when a cutoff value of three or five times the upper limit of the respective reference interval is used (14% for lipase activity and 18% for amylase activity in one study that used a cutoff of three times the upper limit of the reference range).5,18,27 Thus many dogs with pancreatitis may have normal serum activities, and therefore normal serum amylase and/or lipase activities do not rule out pancreatitis.5,36 The low sensitivity of serum amylase and lipase activity assays is at least partially associated with the broad reference intervals for these assays, which are the result of extrapancreatic amylase and lipase activities. A new lipase assay (using the substrate 1,2-O-dilauryl-rac-glycero glutaric acid-[69-methyl resorufin]-ester (DGGR) was recently evaluated and might be more useful for the initial evaluation of dogs suspected of having pancreatitis because of its higher sensitivity (93%) compared with the traditional assays.45 However, the specificity of this assay was very low (53%), limiting the clinical usefulness of this assay.45
Serum lipase activity increases and serum amylase activity decreases in experimentally induced acute pancreatitis in cats.30,46,47 Although well-designed clinical studies are lacking, both serum lipase and amylase activities do not appear to be of any clinical value in the diagnosis of spontaneous feline pancreatitis.10,32,48 Therefore these two tests are not recommended for the diagnosis of pancreatitis in cats.10,48
Trypsin-Like Immunoreactivity
Serum cTLI concentrations increase after experimental induction of pancreatitis in dogs, but rapidly decrease to reference interval concentrations within 3 days of disease induction.40 The sensitivity of serum cTLI for the diagnosis of spontaneous pancreatitis is low (36% to 47%), probably as a result of its short half-life.18,27,28 In addition, although there is strong evidence that trypsinogen is exclusively of pancreatic origin,41 it is believed that it is cleared by glomerular filtration, and serum cTLI concentration can be increased in dogs with glomerular and other renal diseases.28,40 This clearly affects the specificity of the test and complicates the interpretation of increased cTLI concentrations in dogs with azotemia.
In cats with experimentally induced pancreatitis, fTLI concentration increases sharply after induction of pancreatitis, but returns below the cutoff value for pancreatitis within 48 hours.30 fTLI has been evaluated for the diagnosis of spontaneous pancreatitis in cats and several cutoff values have been suggested.33–35 When cutoff values allowing adequate specificity of the assay are used (i.e., 100 µg/L), the sensitivity of fTLI for the diagnosis of pancreatitis in cats is generally low (28% to 33%), with the highest reported sensitivity for this cutoff value being 64%.33–35 In addition, the specificity of fTLI has been questioned, because mildly increased serum fTLI concentrations have been reported in cats with no demonstrable pancreatic disease, but other gastrointestinal disorders (e.g., inflammatory bowel disease or gastrointestinal lymphoma) and azotemia.15,33,35
Diagnostic Imaging
Abdominal Radiography
Conclusive diagnosis or exclusion of pancreatitis is not possible based on abdominal radiography alone.5,9–11,34,43 In the majority of cases of canine and feline pancreatitis abdominal radiographs are normal or reveal nonspecific findings. Despite that, radiography remains a logical initial approach for patients suspected of having pancreatitis because it is relatively inexpensive and useful for the diagnosis and/or ruling out of other differential diagnoses.
In a group of 70 dogs with fatal acute pancreatitis the sensitivity of abdominal radiography was very low (24%).5 Radiographic findings that have been reported for dogs with pancreatitis include an increased soft tissue opacity and decreased serosal detail in the cranial right abdomen, indicating localized peritonitis.5 Other findings may include displacement of the stomach and/or duodenum from their normal positions and gaseous dilation of bowel loops adjacent to the pancreas.5 Abdominal effusion or the presence of an abdominal mass might also be detected. Radiographic findings in cats with pancreatitis are similar to those in dogs.10,11,34,49 In any case, radiography should always be followed by use of more sensitive and specific tests for the definitive diagnosis or exclusion of pancreatitis.
Abdominal Ultrasound
Abdominal ultrasound has been reported to have a relatively high sensitivity of approximately 68% for severe acute pancreatitis in dogs,5 although with increasing equipment quality the sensitivity might have increased since this report.5 Abdominal ultrasound has mainly been assessed in dogs with fatal acute pancreatitis, in which lesions are usually pronounced, but its sensitivity would be expected to be lower in cases with mild or moderate pancreatitis.5 It must be emphasized that a normal pancreas on ultrasound examination does not rule out pancreatitis in dogs.
Ultrasonographic findings in dogs with pancreatitis include hypoechoic areas within the pancreas (possibly indicating necrosis or fluid accumulation), increased echogenicity of the surrounding mesentery (because of necrosis of the peripancreatic fat), enlargement and/or irregularity of the pancreas, dilation of the pancreatic or biliary duct, and abdominal effusion (Figure 60-9).5,50 On occasion, hyperechoic areas of the pancreas can be identified, possibly indicating the presence of pancreatic fibrosis. Cavitary lesions, a thickened duodenum, and biliary obstruction might also be noted.50 If stringent criteria are applied, the specificity of abdominal ultrasonography for canine pancreatitis is considered to be relatively high, although other diseases of the pancreas (e.g., neoplasia, hyperplastic nodules, edema caused by portal hypertension or hypoalbuminemia) may display similar ultrasonographic findings and cannot be differentiated from pancreatitis in many cases.51,52 In a recent study where ultrasonography was performed in 26 animals (both dogs and cats) with suspected gastrointestinal disease, 6 (23.1%) of the animals had ultrasonographic evidence consistent with pancreatitis, while histopathology revealed either a normal pancreas or pancreatic hyperplasia.53 In the same study, there was only a 22% agreement between the ultrasound report and pancreatic histopathology in dogs.53 These data raise concerns regarding the accuracy of ultrasonography in evaluating the canine pancreas and underscore the importance of not overinterpreting ultrasonographic findings.
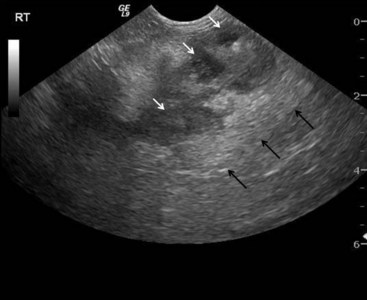
Figure 60-9 Ultrasonographic appearance of the pancreas of a dog with pancreatitis.
(Courtesy Dr. B. Young, Texas A&M University.)
The reported sensitivity of abdominal ultrasonography for the diagnosis of feline pancreatitis is generally low (11% to 35%), with only one study reporting a sensitivity of 67%.11,29,33,49 This high range of sensitivity likely reflects differences in the level of suspicion or the skills of the examiner, the equipment used, and the severity of lesions, and highlights the lack of standardized diagnostic criteria.33,34,49 The low sensitivity of abdominal ultrasonography suggests that many cats with pancreatitis remain undiagnosed if the diagnosis is based solely on ultrasound examination.11,33,49 The sensitivity of abdominal ultrasonography is believed to have increased since the reports mentioned previously as a result of advances in equipment and an increasing level of awareness of the importance of feline pancreatitis, although this has not yet been confirmed. Abdominal ultrasonography has been thought to be relatively specific for the diagnosis of pancreatitis is cats but, similarly to dogs, other diseases (e.g., pancreatic neoplasia, edema) may be associated with similar findings.54 In a recent study, there was an overall agreement of only 33% between the ultrasound report and pancreatic histopathology in cats, and some cats that had ultrasonographic evidence of pancreatitis had no evidence of pancreatitis on histopathology.53 Ultrasonographic findings in cats with pancreatitis are similar to those described in dogs.11,33,49,50,55 It has been suggested that a dilation of the pancreatic duct is suggestive of pancreatitis in cats, but studies have not confirmed this hypothesis.56 In general, feline pancreatitis is often difficult to diagnose by abdominal ultrasound examination and it is important to note that a normal ultrasound examination does not rule out feline pancreatitis.29,33
Overall, abdominal ultrasonography is very useful for the diagnosis of pancreatitis in dogs and cats, especially when performed by an experienced ultrasonographer. Caution should be taken however not to overinterpret ultrasonographic findings. Abdominal ultrasonography is also helpful in detecting possible concurrent abdominal disease in dogs and cats suspected of having pancreatitis. In addition, ultrasound-guided fine-needle aspiration is a useful tool for the diagnosis of pancreatitis and some of its complications (e.g., pancreatic pseudocyst and pancreatic abscess), as well as the management of noninfectious fluid accumulations (e.g. pancreatic pseudocyst).55
Other Imaging Modalities
Although contrast-enhanced computed tomography is an extremely valuable tool for the evaluation of human patients with suspected pancreatitis, initial studies in dogs have not been promising.57 Also, computed tomography performed in cats with histologically confirmed pancreatitis showed disappointing results and currently cannot be recommended.29 Other imaging methods (e.g., endoscopic retrograde cholangiopancreatography, endoscopic ultrasonography), have been used in healthy dogs and cats, in dogs with experimentally induced pancreatitis, and in dogs with gastrointestinal diseases, with varying results. Because of the lack of standardized criteria for the diagnosis of pancreatitis, the complexity of these modalities, their limited availability, and the cost of the equipment, they cannot currently be recommended for the diagnosis of canine or feline pancreatitis.
Pathology
Direct visualization of the pancreas is possible during exploratory laparotomy and laparoscopy. Gross pancreatic lesions suggestive of pancreatitis include peripancreatic fat necrosis, pancreatic hemorrhage and congestion, and a dull granular capsular surface (Figure 60-10).10,18,49 However, gross pathologic lesions may not always be apparent in dogs and cats with pancreatitis.1,10,49
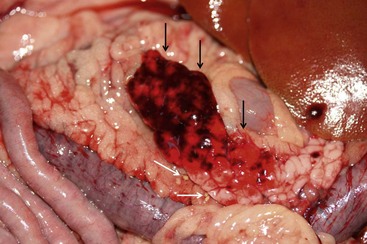
Figure 60-10 Gross Appearance of the pancreas of a dog with acute pancreatitis.
(Courtesy Dr. D. Ajithdoss, Texas A&M University.)
At present, a definitive diagnosis of pancreatitis can only be made by histopathologic examination of the pancreas. Histopathology is also the only way to differentiate acute and chronic pancreatitis. Histopathologic scoring systems for the evaluation of severity of pancreatitis have been proposed for both dogs and cats.1–358 However, histopathologic criteria for the classification of pancreatitis have not been universally standardized in veterinary medicine and substantial confusion exists regarding both classification and terminology of canine and feline pancreatitis, underlying the need for a universally accepted multidisciplinary classification system as is available for humans. The presence of permanent histopathologic changes (such as fibrosis and acinar atrophy) is generally considered suggestive of chronic pancreatitis (Figure 60-11).1,43 Also, the predominant inflammatory cellular infiltrate (neutrophils or lymphocytes) is often used to describe pancreatitis as suppurative or lymphocytic, and some authors consider a suppurative inflammation compatible with acute disease and lymphocytic infiltration compatible with chronic disease (Figure 60-12).10,11 A significant degree of necrosis is usually used to characterize the pancreatitis as necrotizing. It should be noted that some animals can show histopathologic evidence of both suppurative and lymphocytic pancreatitis.
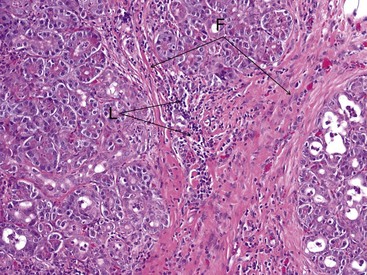
Figure 60-11 Histopathologic appearance of the pancreas of a cat with chronic pancreatitis.
(Courtesy Dr. B.F. Porter, Texas A&M University.)
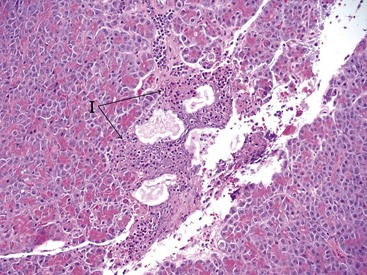
Figure 60-12 Histopathologic appearance of the pancreas of a cat with acute pancreatitis.
(Courtesy Dr. B.F. Porter, Texas A&M University.)
Several limitations are associated with pancreatic histopathology as a definitive diagnostic tool for pancreatitis. First, determining the clinical significance of histopathologic findings may be challenging. In a recent study, 47 (64%) of 73 dogs that presented for necropsy for various reasons had microscopic evidence pancreatitis.1 Similarly, histopathologic lesions of pancreatitis were found in 67% of all cats examined, including 45% of healthy cats.2 Currently, there are no standardized criteria that distinguish microscopic findings leading to clinical disease from those that do not, and it is possible that clinically insignificant pancreatic lesions could lead to a false diagnosis of pancreatitis. At the same time, exclusion of pancreatitis based on histopathology is difficult because inflammatory lesions of the pancreas are often highly localized and can easily be missed.1,2,10,49 Therefore, multiple sections of the pancreas must be evaluated to increase the likelihood of finding microscopic lesions, although this is not always feasible in clinical practice. The absence of histopathologic findings of pancreatitis must be evaluated with caution, especially when only one section of the pancreas has been examined.1,2 Finally, although pancreatic biopsy per se is considered safe, it requires invasive procedures that are expensive and potentially detrimental in patients with pancreatitis that are hemodynamically unstable.53
Cytology
Fine-needle aspiration of the pancreas and cytologic examination is minimally invasive, relatively safe, and can be used for the diagnosis of pancreatitis in both dogs and cats.59 To date no study has evaluated the sensitivity and specificity of this modality for the diagnosis of canine or feline pancreatitis, but the finding of inflammatory cells is considered specific for pancreatitis. Pancreatic acinar cells constitute the majority of the cells found in fine-needle aspiration smears from a normal pancreas (Figure 60-13).59 In patients with acute pancreatitis the cytologic picture is mainly characterized by hypercellularity and the presence of intact and degenerated neutrophils and degenerated pancreatic acinar cells (Figure 60-14). In patients with chronic pancreatitis, small numbers of lymphocytes and neutrophils are usually present, and the specimen is often characterized by low cellularity, possibly as a result of replacement of the normal pancreatic tissue by fibrotic tissue.59
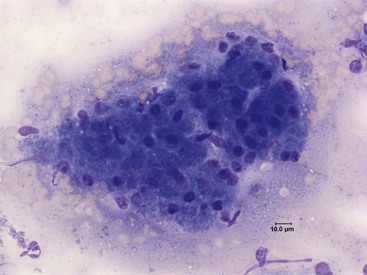
Figure 60-13 Cytologic Appearance of a fine-needle aspirate from a normal canine pancreas.
Acinar cells can be seen in the form of a multicellular cluster. Diff-Quik; magnification 500×.
(Courtesy Dr. P.J. Armstrong, University of Minnesota.)
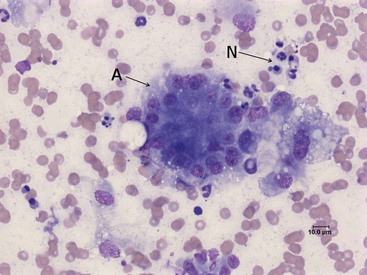
Figure 60-14 Cytologic appearance of a fine-needle aspirate from a canine pancreas with suspected pancreatitis.
(Courtesy of Dr. P.J. Armstrong, University of Minnesota.)
Fine-needle aspiration cytology should be performed either under ultrasonographic guidance or during laparotomy.59 It should be noted that, as for histopathology, highly localized lesions might be missed. Thus negative results are not sufficient to rule out pancreatitis. Fine-needle aspiration cytology might also be useful in differentiating other conditions of the pancreas.
Assessment and Prediction of the Severity of Pancreatitis
Assessment of the severity of human acute pancreatitis is based on the application of standardized severity scores.60 Prediction of the severity of pancreatitis constitutes a very important component of the diagnosis of pancreatitis, because it allows prediction of the likelihood of complications and morbidity, and helps determine the optimal therapeutic plan before the patient enters a critical stage. It is based on a theory that states that the severity of a pancreatitis episode is determined by events that occur within the first 24 to 48 hours of the episode.61 These events are reflected through clinical, clinicopathologic, and imaging findings that can be used to predict the severity of the pancreatitis.61
In veterinary medicine, no well-established and universally accepted severity scores for pancreatitis have been described. Serum PLI and TLI concentrations lack prognostic significance because they correlate poorly with histopathologic severity.18 Currently, severity of canine and feline pancreatitis is determined based on the clinician’s clinical judgment, and typically a diagnosis of severe pancreatitis is made after the animal has entered a critical stage. In general, evidence of systemic complications (e.g., oliguria, azotemia, icterus, severely increased hepatic enzyme activities, hypocalcemia, hypoglycemia, severe hyperglycemia, leukocytosis, shock, or DIC) are considered as indicators of severe disease and a poor prognosis.62–64 However, prediction of the severity of pancreatitis has not been sufficiently studied in dogs and cats. Markers that might prove useful in predicting the severity and/or outcome of a pancreatitis episode are serum C-reactive protein concentrations, serum interleukin-6 concentrations, and plasma and urine trypsinogen activation peptide concentration as well as urine trypsinogen activation peptide-to-creatinine ratio.
Exocrine Pancreatic Insufficiency
Clinical Features
The classical and most common presentation of dogs with EPI involves a chronic history of weight loss, a normal or increased appetite, and loose stools, which is usually characterized by passage of large volumes of semiformed feces. However, it is not uncommon for some dogs with EPI to present with a clinical picture that deviates from the classical picture. In those cases, periods of anorexia, absence of loose stools, occasionally watery diarrhea, or vomiting might be seen. Other possible clinical signs include coprophagia, borborygmus, flatulence, abdominal discomfort, and a poor hair coat. In some cases, EPI may be subclinical and those cases can only be diagnosed with appropriate laboratory testing (see “Trypsin-Like Immunoreactivity” section that follows). Cats with EPI have a similar presentation to that of dogs. In cases where chronic pancreatitis is the cause of EPI, polyuria and polydipsia may be seen as a result of concurrent diabetes mellitus.
Trypsin-Like Immunoreactivity
Serum cTLI is the test of choice for the diagnosis of EPI in dogs. This test is highly sensitive and specific for the diagnosis of EPI, and a positive test (usually defined as <2.5 µg/L) in a dog with compatible clinical signs is sufficient to make a diagnosis of EPI.65 A cTLI result that is well within the reference range is sufficient for excluding EPI, and a normal cTLI result should direct clinicians toward the investigation of other disorders as the cause of the clinical signs observed.65 Single cTLI results within the equivocal range (usually between 2.5 and 5.7 µg/L) in dogs with clinical signs of gastrointestinal disease need to be interpreted with caution.66 In these patients, subsequent retesting of serum cTLI concentration shows either a normal concentration or progression to EPI.66 Therefore, patients with cTLI results in the equivocal range should be investigated for chronic intestinal disease, while reevaluating the cTLI a few weeks later. Some dogs with no clinical signs characteristic of EPI have repeatedly subnormal (<5.7 µg/L) cTLI concentrations.66,67 These dogs have been shown to have subclinical EPI and some are expected to develop clinical EPI in the future.66,67 The time of progression from the subclinical to the clinical stage varies greatly and might be from a few months to years.67 Thus these patients should be closely monitored for the development of clinical signs of EPI and cTLI testing should be repeated every 3 to 6 months.66,67 Finally, because renal disease might increase serum cTLI concentrations and obscure a diagnosis of EPI, reevaluation of nondiagnostic serum cTLI concentrations in azotemic dogs suspected of having EPI is recommended. Similarly, concurrent inflammation might falsely increase the serum cTLI concentration.
Because EPI appears to be less common in cats than in dogs, diagnosis of this disease has been less-well investigated. Similar to dogs, the fTLI test appears to be the most reliable test for the diagnosis of EPI in cats, having a specificity of at least 85%.68 The sensitivity of this assay for the diagnosis of feline EPI has not been evaluated to date. Although there are currently two assays that measure fTLI in serum (one radioimmunoassay that is available in the United States and one enzyme-linked immunosorbent assay that is available in Europe), the analytical validation has only been published for the radioimmunoassay, which is available through the Gastrointestinal Laboratory at Texas A&M University. Similar to dogs, it can be recommended that equivocal serum fTLI concentrations in azotemic cats suspected of having EPI be reevaluated, because renal disease might falsely increase serum fTLI concentrations. The same is true for cats with concurrently increased serum fPLI concentrations indicating residual pancreatic inflammation.
Pancreatic Fecal Elastase
An enzyme-linked immunosorbent assay for the measurement of pancreatic elastase in feces is commercially available and is marketed in Europe (Shebo Biotech, Germany) for the diagnosis of canine EPI.69,70 A recent study reported false-positive results in 23.1% of cases71 and its sensitivity has not been sufficiently evaluated. The fact that this test is easy and quick to perform might make it a reasonable initial approach for dogs with suspected EPI, but as a consequence of its poor positive predictive value a positive test result must be verified by measurement of serum cTLI concentration. This test might also be useful for EPI cases that are a result of pancreatic duct obstruction. However, to date such a case has only been anecdotally reported in the veterinary literature.
Other Tests
Serum amylase and lipase activities have been shown to have no value in the diagnosis of EPI in either dogs or cats.21,41,72 Canine PLI concentrations are low or undetectable in most dogs with EPI, but some overlap between healthy dogs and dogs with EPI does exist, making this test inferior to cTLI for the diagnosis of EPI.21 However, cPLI might be used to diagnose isolated pancreatic lipase deficiency, a rare form of EPI, where serum cTLI and other pancreatic zymogen concentrations are expected to be normal.73 Commercial assays for the measurement of serum PLI concentration (Spec cPL and Spec fPL) are not useful for the diagnosis of EPI in dogs and cats, respectively, because they have been optimized to detect changes in the higher ranges of their respective working ranges.
Measurement of the fecal proteolytic activity has been used in the past for the diagnosis of EPI in dogs and cats, but are now only used for species for which a TLI assay is not available.74,75 A plethora of other tests, including microscopic examination of feces and the bentiromide absorption (benzoyl-tyrosyl-paraaminobenzoic acid [BT-PABA]) test, have also been described for the diagnosis of EPI in the past. However, these tests often give false-positive and/or false-negative results and many of them are impractical, expensive, or of limited availability. Thus none of these tests are recommended for the diagnosis of canine or feline EPI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree