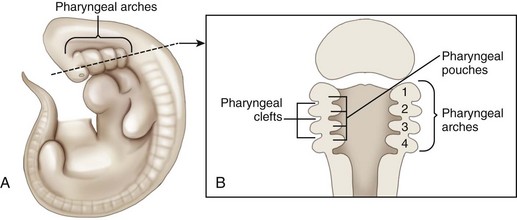
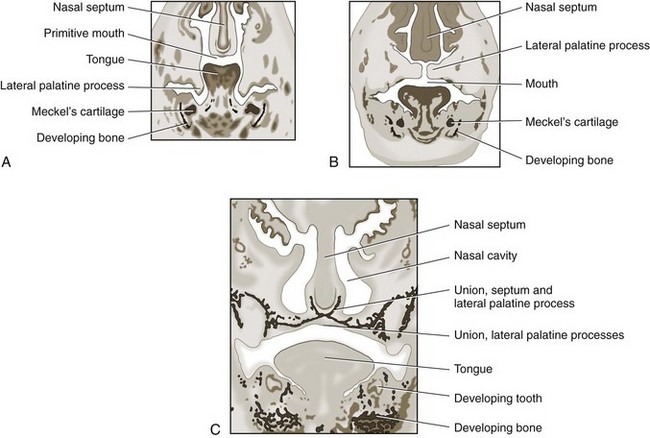
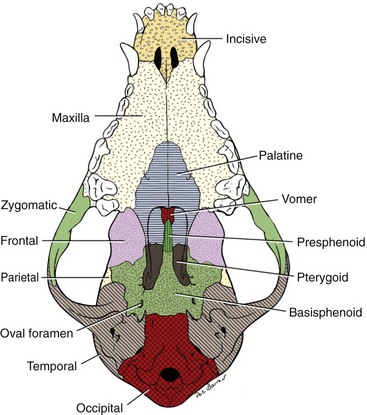
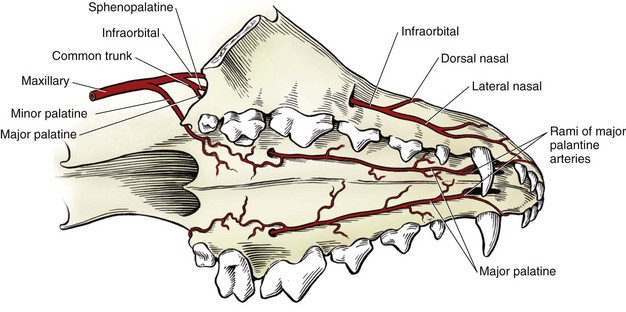
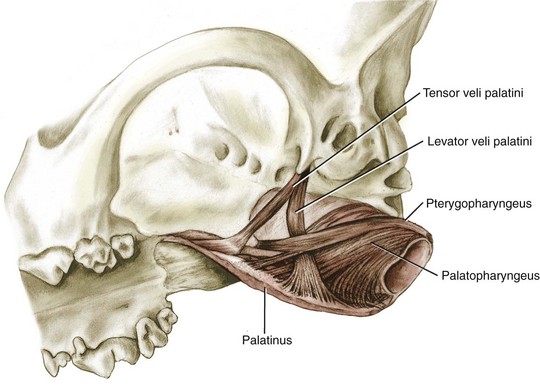
Chapter 100 Development of the head begins during the fourth week of gestation as neural crest cells migrate between the surface ectoderm and foregut endoderm as part of the formation of six pairs of pharyngeal arches (Figure 100-1).48 The first pair of pharyngeal arches gives rise to the ventral mandibular and dorsal maxillary prominences bilaterally, which surround the stomodeum, an invagination of ectoderm that is the precursor to the oral cavity. The structures contributing to the formation of the lip and primary palate in dogs and cats are disputed. In other species, the medial nasal and maxillary prominences (also referred to as process in several texts) fuse to form the lip and primary palate.9,15,48,49 Based on a morphologic study of dogs with clefts of the lip and primary and secondary palate, some authors suggest that the medial nasal prominences do not contribute significantly to lip formation66 or that the primary palate in dogs and cats is formed only by the maxillary processes.82 The secondary palate is formed from bilateral palatal shelves that grow out from the maxillary processes. Initially, the palatal shelves grow vertically down on either side of the developing tongue.22 These palatal shelves then elevate rapidly above the tongue and join with each other and the developing nasal septum (Figure 100-2). The midline epithelium from each palatal shelf degrades, resulting in a fused secondary palate.16 The nasal epithelium differentiates into pseudostratified ciliated columnar epithelial cells, and the oral epithelium differentiates into stratified squamous cells under the influence of complex cytokine signaling from the underlying mesenchyme causing gene expression through various second messenger systems.22 The hard palate is composed of the palatine, maxillary, and incisive bones and the palatal mucoperiosteum (Figure 100-3). The stratified squamous oral epithelium of the hard palate contains six to 10 transverse ridges and depressions. The incisive papilla opens at or just rostral to the first transverse ridge on the midline. Incisive ducts adjacent to the papilla communicate with the vomeronasal organ and the nasal cavity. The major palatine foramina are located medial to the maxillary fourth premolar teeth on either side of the hard palate. One or more smaller minor palatine foramina may be located caudal to each major palatine foramen. The major palatine artery exits through the major palatine foramen, coursing rostrally and supplying the hard palate. Several rami enter the palatine fissure, and one ramus courses between the maxillary canine and third incisor tooth to anastomose with branches of the infraorbital artery (Figure 100-4). The major palatine branch of the maxillary division of the trigeminal nerve also runs through the major palatine foramen and provides sensory innervation to the oral side of the hard palate.20,21 The soft palate is continuous with the hard palate and extends just caudal to the last maxillary molar teeth in most nonbrachycephalic breeds. It is composed of a stratified squamous oral epithelium rich in palatine glands, the palatine muscles, and a nasal epithelial surface. Blood to the soft palate is principally supplied by the minor palatine arteries and drained by the extensive palatine plexus that lies lateral to the palatine muscles. The minor palatine branch of the maxillary division of the trigeminal nerve provides sensory innervation to the soft palate. The glossopharyngeal and vagus nerves supply the pterygopharyngeal and palatopharyngeal muscles.21 The soft palate muscles include the palatinus and the levator and tensor veli palatini. The palatinus muscle runs from the palatine process of the palatine bone to the caudal border of the soft palate and shortens the soft palate rostrocaudally when it contracts. The tensor veli palatine muscle arises from a bony process rostral to the tympanic bulla and passes ventrally over the hamular process of the pterygoid bone, where it becomes tendinous, and inserts diffusely on to the palatine aponeurosis. It stretches the soft palate between the pterygoid bones. The levator veli palatine muscle has a similar origin and then courses caudally and ventrally to insert onto the caudal half of the soft palate. It elevates the caudal soft palate, helping protect the nasopharynx during swallowing and vomiting (Figure 100-5).18,21 The soft palate has two functions during swallowing: (1) stimulation of sensory nerves in the palate is part of the mechanism that triggers swallowing,51,81 and (2) closure of the intrapharyngeal opening during swallowing and vomiting prevents swallowed food, liquid, and vomitus from entering the nasopharynx and being subsequently aspirated. The caudal soft palate is elevated dorsally by the paired levator veli palatine muscles to close the intrapharyngeal opening. In addition, the palatopharyngeus muscles, which originate from the palate and insert onto the dorsal pharyngeal fascia, draw the caudal soft palate, palatopharyngeal arches, and caudal pharynx together during swallowing, thus closing the opening of the nasopharynx.18 The muscles of the soft palate are also likely involved in the physiologic response to increasingly negative upper airway pressures and hypoxic hypercapnia.80 Increased phasic inspiratory, phasic expiratory, and tonic activity of the palatine muscles may stiffen the soft palate during periods of increased respiratory activity to decrease airway resistance.79,80 In humans, contraction of the palatine muscles plays a role in partitioning airflow.61 Similar palatine muscular activity may determine if a dog is nose breathing or mouth breathing at any given time. Palate defects are either present at birth (congenital) or are acquired after birth. Congenital lip and palate defects in cats and dogs can be inherited or are a sequela of intrauterine trauma or stress.27 Incomplete fusion of maxillofacial structures during fetal development may result in uni- or bilateral clefts of the upper lip, lateral area of the most rostral hard palate, midline of the hard and soft palate, and lateral area of the soft palate. Rarely, the soft palate may be markedly reduced in length (hypoplasia). Growth of palatine portions of facial bones in broad-headed fetuses may not always successfully keep pace with the growth of the head; thus, brachycephalic breeds tend to be at higher risk of developing defects of the primary and secondary palates. Causes of palate defects acquired after birth include chronic infections (e.g., severe periodontal disease, osteomyelitis or osteonecrosis), trauma (e.g., high-rise syndrome, motor vehicle trauma, electric cord injury, gunshot trauma, animal bites, foreign body penetration, pressure wounds secondary to malocclusion), neoplasms, and surgical and radiation therapy.3 As in children, unilateral cleft lips in cats and dogs are more frequently on the left side. Cleft lips may be associated with abnormalities of the secondary palate, which are rarely visible externally. Hard palate clefts are almost always in the midline and are usually associated with a midline soft palate cleft. Soft palate clefts without hard palate clefts may occur in the midline or are unilateral. An association between congenital unilateral defects or hypoplasia of the soft palate and middle ear pathology was reported in dogs.85 According to the stage of development and severity of the cause, other physical or neurologic abnormalities may be present. Clefts can occur if the intrauterine insult (trauma; stress; corticosteroids; antimitotic drugs; nutritional, hormonal, viral, and toxic factors) occurs at a very specific time in fetal development (25th to 28th day in dogs).82 Management of patients with defects of the secondary palate usually requires nursing care by the owner, which includes tube feeding to avoid aspiration pneumonia. Most procedures for correction of congenital palate defects are performed on animals between 3 and 4 months of age. Surgery before 2 months of age is challenging because the soft tissues are delicate and friable in very young animals. Postponing surgery until after 5 months of age may result in a wider cleft as the animal grows and in compounded management problems, which are not desirable. An oronasal fistula is caused by loss of incisive or maxillary bone typically associated with severe periodontal disease or tooth extraction.4,76 Fresh midline clefts of the hard palate are often seen in cats with a history of motor vehicle trauma or falling from a height. Electric cord injury occurs most often in young animals, and neurogenic pulmonary edema is an immediate life-threatening concern. It may take several days before the extent of local injury is clearly defined. Necrosis of the hard palate is common. Similarly, it is often best to wait and treat gunshot trauma patients conservatively until necrotic tissues have sloughed. This approach allows determination of viable tissues available for definitive repair at a later time. Palatal defects from dog bites, foreign body penetration, and maloccluding teeth are carefully debrided before surgical repair. In all cases, the cause of the defect must be removed before repair.27 Except for being externally visible, cleft lips rarely result in clinical signs beyond mild local rhinitis, and repair may be performed for purely aesthetic reasons.58 Clinical signs and history associated with congenital secondary palate defects include failure to create negative pressure for nursing, nasal discharge (drainage of milk from the nares during or after nursing), coughing, gagging, sneezing, nasal reflux, tonsillitis, rhinitis, laryngotracheitis, aspiration pneumonia, poor weight gain, and general unthriftiness.27 An acute oronasal fistula after tooth extraction may be diagnosed by direct visualization of the nasal cavity and epistaxis at the ipsilateral nostril. Sneezing and ipsilateral nasal discharge are common clinical signs of a chronic oronasal fistula. A defect in the area of a missing tooth (often in the maxillary canine tooth region) that communicates with the nasal cavity may be noted on oral examination.27 Cats with traumatic cleft palate usually present with bilateral epistaxis or dried blood at the nostrils, a visible malalignment along the midline of the maxillary dental arch, and a midline hard palate cleft with torn palatal mucoperiosteum.56 Hard and soft palate defects acquired after birth are often associated with similar clinical signs as with congenital defects of the secondary palate. The further caudal and the larger the defect, the more severe become the clinical signs. Surgical goals include closure of well-vascularized and tension-free tissues separating the oral and nasal passages. The choice of technique used depends on the age and systemic health of the patient, the viability and integrity of local tissues, the location and size of the defect, the amount of tissue available for flap procedures, and the oral surgeon’s preference.72 The best chance of success is usually with the first procedure. Teeth at the surgical site and those that could traumatize flaps should be extracted 6 to 8 weeks before definitive palate defect repair. It is of paramount importance that the blood supply to the flaps is retained. There is often considerable hemorrhage during palate surgery because of the rich blood supply of the palatal tissues involved; however, use of electrosurgical equipment should be avoided for hemostasis. Digital pressure with gauze sponges is often sufficient to control bleeding. Flaps should be handled as carefully as possible (with stay sutures at their edges) and be at least 1.5 times larger than the defect they will cover. Connective tissue surfaces or large cut edges should be sutured together, and a two-layer closure with synthetic absorbable monofilament should be used, if practical. Closure under tension must be avoided, and suture lines should preferably not be located over a void.27 Numerous approaches may be used for repair or closure of palate defects.68 Surgical repair may be accomplished with a variety of flaps harvested from oral, pharyngeal, and nasal mucosa or the skin, with the choice of technique depending on the location, size, and shape of the defect. Flaps can be classified based on the location of the donor site (local or distant), attachment to donor site (pedicle or free; it has also been suggested that a tissue that is free of the donor site attachment should be termed a graft rather than a flap), tissue to be transferred (e.g., mucoperiosteal, myocutaneous, or myoperitoneal), blood supply (random or axial), and direction and orientation of transfer (advancement, rotation, transposition, and overlapping). Owners should always be warned that multiple procedures might be required to completely close a palate defect. Nonsurgical closure of palate defects involves the use of prosthetic devices. In the case of congenital defects of the primary palate, attempts to close the lip and most rostral hard palate defects by simple sliding procedures are rarely successful because there is no connective tissue bed to support the flaps. The floor of the nasal vestibule and most rostral hard palates are reconstructed by creating flaps of oral and nasal soft tissue or flaps that are harvested from oral soft tissue only. This is often complicated by the presence of teeth in the incisive bone and maxilla; removal of one or more incisors and the canine tooth on the affected side before lip and palate surgery will facilitate flap management. Repair is achieved with advancement, rotation, transposition, or overlapping flaps followed by reconstructive cutaneous surgery to provide symmetry.35,39 The overlapping flap technique is preferred for midline clefts of the hard palate (Figure 100-6).34 There is less tension on the suture line, the suture line is not located directly over the defect, and the area of opposing connective tissue is larger, which results in a stronger scar. It provides more reliable results compared with the medially positioned flap technique, the latter of which is more useful for repair of traumatic midline clefts of the hard palate in cats (Figure 100-7).58 Figure 100-6 Overlapping flap for repair of a cleft of the hard palate. A, Incisions are made in the mucoperiosteum to the bone along the dental arch about 1 to 2 mm away from the teeth and to the rostral and caudal margins of the defect on one side and at the medial margin of the defect on the other side. B, A periosteal elevator is used to create an overlapped flap on one side and an envelope flap on the other side. The major palatine artery must not be transected during flap elevation. When the artery is identified at the connective tissue side of the overlapped flap, careful dissection close to it will release it from surrounding tissue to accommodate the rotation of this flap. C, The overlapped flap is inverted at its base, turned, and secured under the envelope flap with horizontal mattress sutures so that large connective tissue surfaces are in contact. (Reproduced from the BSAVA manual of canine and feline head, neck and thoracic surgery (2005), edited by Daniel Brockman and David Holt. Reproduced with permission from Samantha J. Elmhurst, BA Hons., www.livingart.org.uk.) Figure 100-7 Medially positioned flaps for repair of a congenital cleft of the hard palate. A, Incisions are made in the mucoperiosteum to the bone at the defect margins and along the dental arch about 1 to 2 mm away from the teeth on either side. B, A periosteal elevator is used to carefully undermine the two flaps. C, The flaps are moved medially and sutured to each other. (Reproduced from the BSAVA manual of canine and feline head, neck and thoracic surgery (2005), edited by Daniel Brockman and David Holt. Reproduced with permission from Samantha J. Elmhurst, BA Hons., www.livingart.org.uk.) Incisions are made in the mucoperiosteum to the bone along the dental arch about 1 to 2 mm away from the teeth and to the rostral and caudal margins of the defect on one side, forming an overlapped flap, and at the medial margin of the defect on the other side, forming an envelope flap. Both flaps are carefully undermined with a periosteal elevator. The major palatine artery exits the major palatine foramen at the palatine shelf of the maxilla about 0.5 to 1.0 cm medial to the maxillary fourth premolar (more rostral in cats than in dogs) and must not be transected during flap elevation. When the artery is identified at the connective tissue side of the overlapped flap, careful dissection close to it will release it from surrounding tissue to accommodate the rotation of this flap. The envelope flap is also undermined with a periosteal elevator on its medial margin to create a pocket of space for the overlapped flap. The overlapped flap is inverted at its base so that its periosteal surface is exposed and secured under the envelope flap with horizontal mattress sutures so that large connective tissue surfaces are in contact. Granulation and epithelialization of exposed bone generally are completed in 3 to 4 weeks.58 The medially positioned flap technique is often used for repair of midline clefts of the soft palate (Figure 100-8).27,58 Incisions are made along the medial margins of the defect to the level of the caudal end of the tonsils. Palatal tissues are separated with blunt-ended scissors to form a dorsal (nasopharyngeal) and ventral (oropharyngeal) flap on each side. The two dorsal and the two ventral flaps are sutured separately in a simple interrupted pattern to the midpoint or caudal end of the palatine tonsils.27,58 A bilateral overlapping flap technique for a midline soft palate cleft has also been described.23 Figure 100-8 Medially positioned flap for repair of a cleft of the soft palate (note that repair of a cleft of the hard palate is already completed). A, Incisions are made along the medial margins of the defect to the level of the caudal end of the tonsils, and the palatal tissues are separated with blunt-ended scissors to form a dorsal (nasopharyngeal) and ventral (oropharyngeal) flap on each side. B, The two dorsal and the two ventral flaps are sutured separately in a simple interrupted pattern to the midpoint or caudal end of the palatine tonsils (note that suturing of the dorsal flaps is already completed). C, Repair of the clefts of the hard and soft palates is completed. (Reproduced from the BSAVA manual of canine and feline head, neck and thoracic surgery (2005), edited by Daniel Brockman and David Holt. Reproduced with permission from Samantha J. Elmhurst, BA Hons., www.livingart.org.uk.)
Palate
Embryology
Anatomy
Physiology
Palate Defects
Pathophysiology
Clinical Signs and Diagnosis
Treatment
Repair of Rostral Defects
Overlapping Flap Technique for Hard Palate Repair
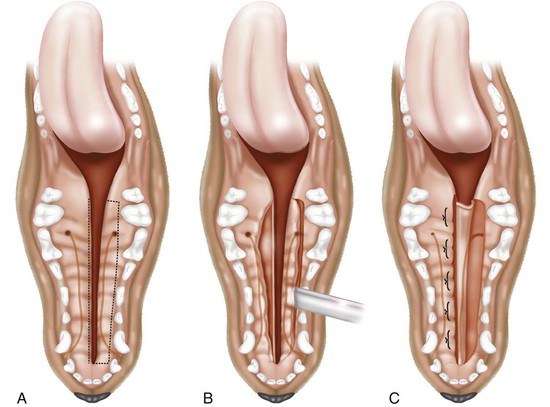
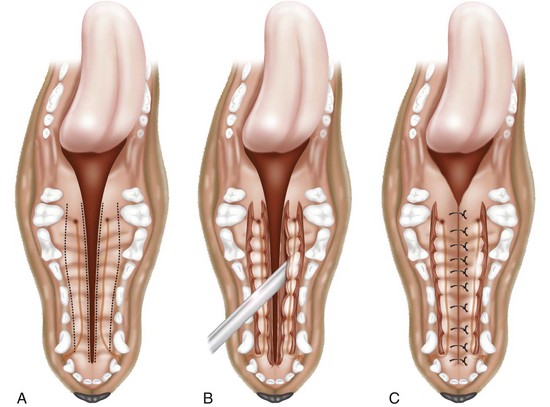
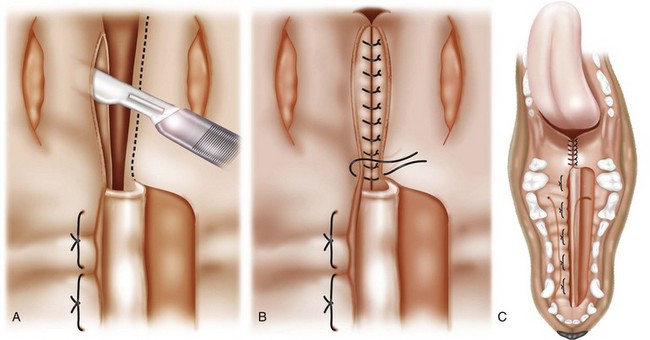
Medially Positioned Flap Technique for Soft Palate Repair
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Palate
Only gold members can continue reading. Log In or Register to continue