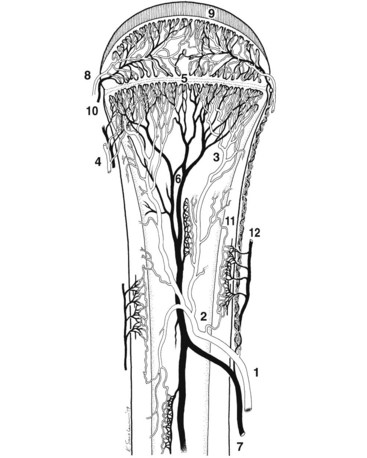
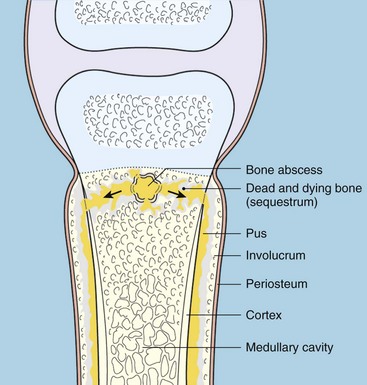
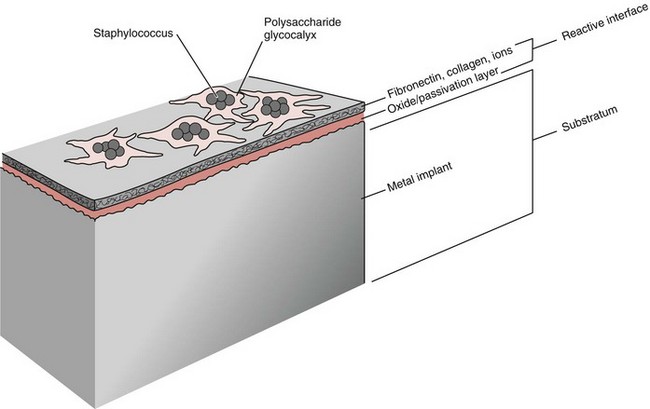
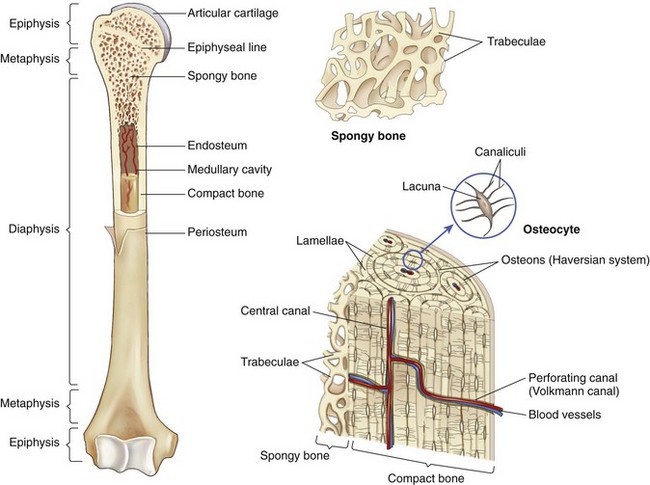
Chapter 48 Osteomyelitis, an inflammatory condition of bone, is usually considered to be an infectious process caused by an infectious agent such as bacteria or fungi. The incidence of osteomyelitis is low because normal bone is highly resistant to colonization and subsequent infection. A negative event, such as trauma, the presence of foreign bodies (surgical implants or prostheses), or inoculation with infective agents that can produce biofilms or that can preferentially adhere to implants, must occur to create osteomyelitis.10 In veterinary medicine, osteomyelitis has been classified by the route the infectious agent takes to enter the site of infection (hematogenous or posttraumatic) based on the time of disease onset (acute, subacute, or chronic).7 Timelines are not always completely clear, but in general acute osteomyelitis develops within 2 weeks, subacute within several months, and chronic after a few months. The current classification is based, in part, on the Waldvogel staging system,51–53 which uses cause of the infection (hematogenous spread versus direct inoculation or contiguous spread from soft tissues). More recently, another staging system for long bone osteomyelitis, defined by Cierny and Mader,9 has been used in human medicine but not in veterinary medicine. It is based on a combination of anatomic factors (medullary, superficial, local, or diffuse) and physiologic considerations (normal host, systemic, or local compromise). Regardless of the classification scheme, rapid diagnosis and aggressive treatment are paramount in the management of osteomyelitis. Bone is a complex tissue (Figure 48-1) that can be divided into regions: diaphyseal, metaphyseal, and epiphyseal, each with a periosteal and endosteal layer. Microscopically, bone can be divided into compact and trabecular components. Compact bone consists almost entirely of extracellular matrix. Osteoblasts deposit this matrix in the form of thin layers known as lamellae. In the process of deposition of the matrix, osteoblasts become encased in small hollows within the matrix, the lacunae. Osteocytes have several processes, which extend from the lacunae in small channels known as canaliculi. Canaliculi arising from one lacuna may anastomose with those of other lacunae and, eventually, with larger, vessel-containing canals within the bone. Compact bone lamellae form concentric rings around larger longitudinal canals known as Haversian canals. Haversian canals typically run parallel to the surface and along the long axis of the bone. Haversian canals generally contain capillaries and nerve fibers. A second system of canals, called Volkmann’s canals, penetrates the bone more or less perpendicular to its surface. These canals establish connections of the Haversian canals with the inner and outer surfaces of the bone. Beneath the periosteum and endosteum, a few lamellae can be found. In trabecular bone, the matrix is also deposited in the form of lamellae. However, lamellae in trabecular bone do not form Haversian systems. Lamellae of trabecular bone are deposited on preexisting trabeculae, depending on local demands on bone rigidity. Figure 48-1 Cortical and cancellous bone structure. Note the relationship of the lamellae to the osteon structure in cortical bone and the absence of the Haversian system in cancellous bone trabeculae. (Modified from http://www.cliffsnotes.com/study_guide/Bone-Structure.topicArticleId-22032,articleId-21902.html.) The blood supply varies with different types of bone, but blood vessels are especially rich in areas that contain bone marrow. In the long bones, the diaphyseal nutrient artery is the most important arterial supply (Figure 48-2). These arteries divide into ascending and descending branches and supply the inner two thirds of the cortex and medullary cavity. Numerous metaphyseal and epiphyseal arteries supply the ends of bones. These blood vessels mainly arise from the arteries that supply the adjacent joint, anastomose with the diaphyseal capillaries, and terminate in bone marrow, cortical bone, trabecular bone, and articular cartilage. In growing bones, these arteries are separated by the physis. It has also been shown that capillaries in the metaphyseal vascular plexuses are discontinuous. Furthermore, ingrowth of these metaphyseal vascular plexuses occurs by extension of “spourts” into chondrocytic lacunae, producing temporary gaps in these vessel endothelial walls.1,36 The periosteal arterioles supply the outer approximately one third of cortical bone. Large irregular bones, short bones, and flat bones receive a superficial blood supply from the periosteum, as well as frequently from large nutrient arteries that penetrate directly into medullary bone, and these systems anastomose freely. At the edge of this ischemic bone is a reactive hyperemia that is associated with increased osteoclastic activity. This osteoclastic activity produces bone loss and localized osteoporosis.26 Additionally, periosteal irritation occurs, and the osteoblasts that are present react by producing new bone (Figures 48-3, 48-4, and 48-5). The amount and type of periosteal reaction are characterized by the aggressiveness of the infection; less aggressive infection pushes the periosteum away from the bone slowly and results in thickening of the bone. More aggressive infection results in a lamellated (onion skin) appearance wherein the bone is laid down in layers. Aggressive infection causes the periosteum to be rapidly pushed away from the bone and leads to the development of calcified streaks perpendicular to the bone (Figure 48-6).29 Hematogenous osteomyelitis usually occurs in the metaphyseal region of long bones (see Figure 48-4). The prevailing theory on why the metaphyseal region is susceptible to the development of hematogenous osteomyelitis is based on the fact that discontinuous capillaries (capillaries that have gaps between adjacent endothelial cells and an incomplete basement membrane which also has gaps in it) are present in the metaphyseal region which may allow easier passage of the bacteria into the adjacent tissues. Additionally, during bone growth, capillary buds in the metaphyseal region lack a basement membrane and have a discontinuous endothelium. Thus during bacteremia, bacterial translocation and embolization occur through the endothelial gaps into an area that is relatively inaccessible to the host immune response of inflammatory cells.15,31 Additionally, it has been stated (without supportive data) that a “sluggish” circulation is present near the growth plate, which favors deposition and proliferation of bacteria.21,30,45,46 However, this theory may ignore other aspects of host defense mechanisms in metaphyseal bone that may play a role in the development of infection. Experimental data call into question some of these previous ideas and points to the potential of alteration of immune response to the invading bacteria through both the downregulation of T-cell immunity and cytokine production.54,55 A final possible contributor is the apparent absence of leukocytes from developing primary spongiosa; thus bacterial invasion at these sites is opposed only by tissue-based macrophages. Clearly, the pathophysiology of acute hematogenous osteomyelitis is not completely understood. Yet, regardless of the exact mechanism of bacterial “seeding” of the metaphyseal region, once the infection is established, the aforementioned inflammatory process ensues with the production of an ischemic region of infected necrotic and devitalized tissue. An interesting species anatomic difference is that, unlike other species, dogs and cats lack transphyseal vasculature at birth; thus concurrent joint infections are very uncommon.18 Osteomyelitis initiated by direct inoculation (posttraumatic) is the most common bone infection seen in companion animal practice. An understanding of the pathophysiology of posttraumatic osteomyelitis is vital to the clinician who is attempting to treat it. Fundamental factors associated with the pathophysiology of these infections include the virulence of the infecting organism, the immune status of the patent, the bone affected (including location on the bone), and other comorbidities such as systemic trauma, sepsis, and major organ dysfunction. In terms of causative organisms, staphylococcal species are reported in up to 60% of cases of bacterial osteomyelitis in dogs and cats.25 Staphylococcus intermedius is the most common, although other Gram positive organisms are occasionally involved. Gram negative organisms, including Escherichia coli, Pseudomonas, Proteus, and Klebsiella species, have also been described.18,25,27 Other infectious agents, including Blastomyces, Coccidioides, Cryptococcus, and Histoplasma isolates, are mainly fungal. However, mycotic infections are usually hematogenous in origin. Factual data supporting initiation of osteomyelitis by viral agents are limited. Identification of anaerobic bacterial involvement has increased, most likely as the result of improved methods of culturing. Consequently, polymicrobial infections, including aerobic and anaerobic combinations, are now more commonly diagnosed. The exact rate of anaerobic infection by a singular agent or within a poly-infection is not known.6,7,18 Bone infection involves a deleterious interaction between the host and the offending causative agent. Bacteria not only must contaminate, but also must colonize the bone and surrounding tissues to cause an infection. Thus osteomyelitis occurs when vascular compromise and subsequent tissue ischemia are coupled with bacterial contamination. Tissue trauma (both traumatic and surgically induced) and subsequent vascular compromise can account for all of the aforementioned factors that predispose bone to infection. Thus the importance of tissue trauma in the development of posttraumatic osteomyelitis cannot be overemphasized. It is interesting to note that in human cases of posttraumatic osteomyelitis, inflammatory cells, (PMNs) infiltrate the infected site, but despite local activation are unable to clear the bacteria, presumably because of biofilm formation. It is hypothesized that during the ineffective attempt to phagocytose, PMNs release cytotoxic and proteolytic entities that in turn contribute to the progression of tissue injury and ultimately to osteolysis.50
Osteomyelitis
Classification
Anatomy
Pathophysiology
Hematogenous Osteomyelitis
Posttraumatic Osteomyelitis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree