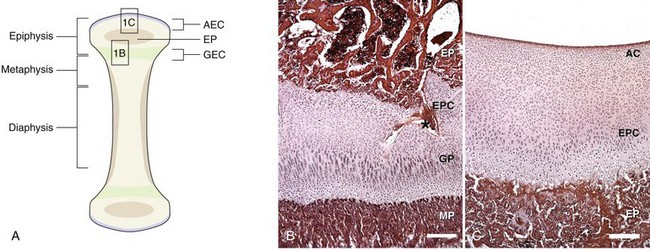
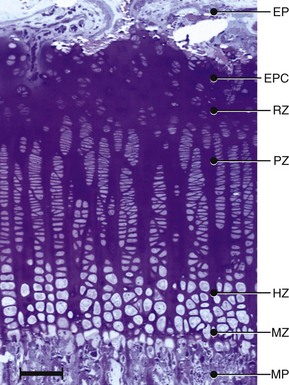
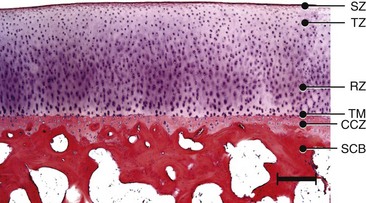
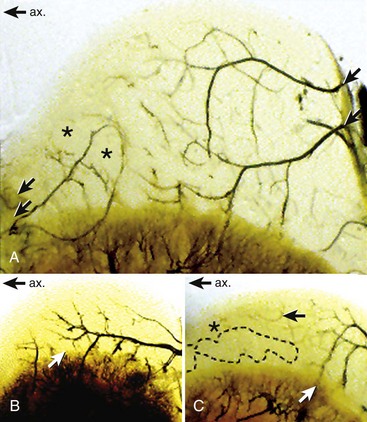
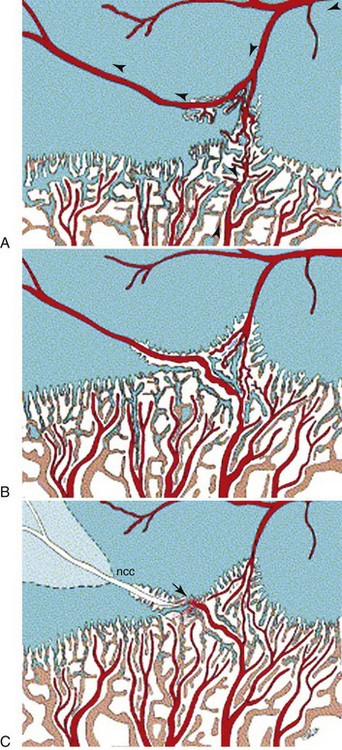
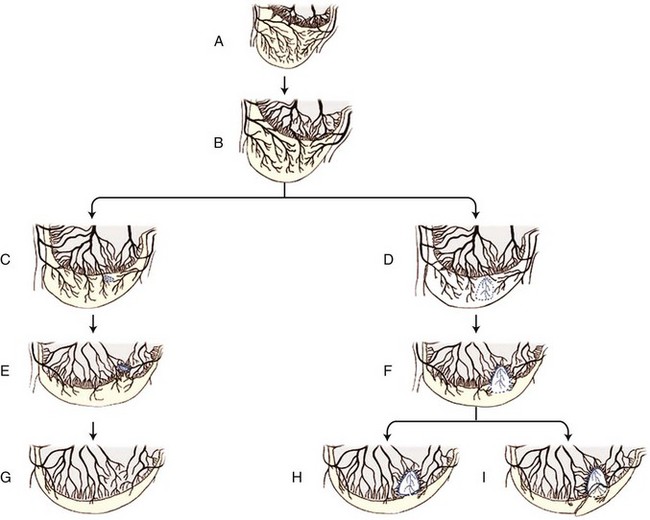
Chapter 73 Osteochondrosis is a term used to indicate a common developmental condition of growth cartilages in skeletally immature animals and man. Specifically, it describes a disorder of the ordered process of endochondral ossification, a process whereby growth cartilage in epiphyses and growth plates is gradually turned into bone through a sequence of matrix mineralization, chondrocyte death, vascularization, and ossification.107,165 Previous studies have variously described osteochondrosis as a focal53 or generalized107,108,124 disturbance of endochondral ossification. A generalized disease process has more recently been refuted, and based on the localized nature of the initial lesions, often at predictable sites, osteochondrosis has been described as a focal or multifocal disorder, often with a bilaterally symmetric distribution.35,165 Given that endochondral ossification occurs in the growth cartilages at both the epiphyses and the growth plates (also referred to as the metaphyseal growth plate or physis108), a number of authors have argued that osteochondrosis occurs and manifests clinically at both locations.107,111,165 Others have provided a contrary view by suggesting that in dogs (and presumably cats), the term osteochondrosis should be reserved for conditions of the articular surfaces of the shoulder, elbow, stifle, and hock joints, and should exclude conditions of the growth plates of long and cuboidal bones.81,144 Some authors have expanded the term to include conditions of the axial skeleton.32,33,92,136 See Table 73-1 for a summary of generally accepted and presumed manifestations of osteochondrosis. A further confounding aspect of this disease is the difficulty involved in positively relating specific proposed clinical manifestations of osteochondrosis to the initial disorder of endochondral ossification. This occurs because clinical lesions, for example, the chondral or osteochondral fragments that are studied, represent a chronic manifestation of the disease process, whereas early lesions are concealed by reparative processes.35,165 A recently proposed method of classifying osteochondrosis of the articular–epiphyseal cartilage complex is based on the disease stage.167 This system uses the terms osteochondrosis latens to indicate an early, microscopic lesion; osteochondrosis manifesta to describe subclinical lesions that are macroscopically and radiologically apparent; and osteochondrosis dissecans to describe the disease if attached or loose cartilage flaps are present and typically result in clinical signs. Data from veterinary teaching hospitals suggest that articular osteochondrosis was diagnosed in approximately 3.7% of all dogs that were presented with an orthopedic problem, translating into an incidence of 8.1 cases per 1000 patients.69 Approximately 9% of dogs younger than 1 year of age that were presented with an orthopedic problem were diagnosed with osteochondrosis.2 Male dogs are more commonly affected than female dogs. Epidemiologic and genetic studies have indicated strong breed predispositions, particularly for large- and giant-breed dogs, suggesting genetic etiologic components for each of the different manifestations of osteochondrosis.82,134,161 Disease prevalence and breed predispositions differ between disease manifestations and will be further discussed in pertinent chapters. Additional data from veterinary teaching hospitals indicate that only 0.001% of 8909 cats presented with an orthopedic problem were diagnosed with osteochondrosis.2 In addition, only a few cases of feline osteochondrosis have been reported in the veterinary literature.17,117,122 Thus, feline osteochondrosis is very uncommon. Canine skeletal development and feline skeletal development follow a similar pattern and temporal sequence. During embryonic development (days 19 to 35 after fertilization), the first evidence of the developing skeleton, with the exception of the skull bones, is the so-called condensation of mesenchymal cells in the locations of future bones of the axial and appendicular skeleton.36,102,103 The mesenchymal bone models transform into cartilage, enlarge by forming new cartilage in the epiphyses and growth plates, and convert the newly formed cartilage into mature bone via endochondral and intramembranous ossification (see later).36,102,103,105 Joints form between developing bones by the processes of segmentation and cavitation; this is followed by the formation of intra-articular structures, including articular cartilage.105 Longitudinal growth of long bones originates in their epiphyses and growth plates. In most long bones, growth plates contribute approximately 75% to 80% of the final bone length, whereas epiphyseal growth centers contribute approximately 20% to 25% (Figure 73-1). In dogs, most longitudinal bone growth takes place between 12 and 26 weeks of age. During this period, growth and mineralization of all epiphyseal growth centers cease at predetermined times, and growth plates have their most rapid rate of longitudinal bone growth and endochondral ossification. At the end of this period, cartilage only persists between the epiphysis and the metaphysis and at the articular surface covering the epiphyses.74 Thereafter, growth plate–associated bone length growth continues at a much slower rate until it ceases completely. At cessation of growth, growth plates mineralize at predetermined times (Table 73-2). The rate of skeletal development and the final size of bones vary widely among canine and feline breeds. Table • 73-2 Ossification Center Appearance and Growth Plate Fusion of Selected Bones in Immature Dogs115,142 Growth plates enlarge through the formation of new cartilage. The volume of newly formed cartilage is a function of chondrocyte proliferation, chondrocyte enlargement, and matrix formation.9,13,158 The cartilage volume increase will mainly result in bone length growth by pushing the articular–epiphyseal complex away from the metaphysis, because circumferential expansion of a growth plate is constrained by the perichondral ring of Lacroix.3,29 Simultaneously, the newly formed cartilage is converted into bone at the junction of the growth plate and the metaphysis via the process of endochondral ossification.29 The growth plate is sandwiched between epiphysis and metaphysis, and may be divided into four zones: the resting zone, the proliferative zone, the hypertrophic zone, and the mineralization zone (see Figures 73-1, B and 73-2).3,74 The resting zone is juxtaposed to the epiphysis and is penetrated by chondro-epiphyseal blood vessels (cartilage canals); this is the only vascularized zone of the growth plate. Here, chondrocytes are small, randomly oriented, and scattered irregularly throughout the matrix; they are primarily stem cells that divide at a slow rate and daughter cells that will start the columns that originate in the proliferative zone. In the proliferative zone, chondrocytes are flat and relatively small and are organized in columns; these chondrocytes divide, slowly enlarge, and synthesize matrix. Columnar organization is continued in the hypertrophic and mineralization zones. Chondrocytes in the hypertrophic zone are spheroid and relatively large; they rapidly enlarge (swelling) and continue synthesis of the matrix. In the mineralization zone, chondrocytes have obtained their final size and shape; here, newly formed matrix mineralizes and chondrocytes die abruptly by the process of apoptosis. Changes in the morphology of the chondrocytes and in the volume and composition of their matrix within the columns represent temporal alterations that take place throughout the life span of the chondrocyte.3,12,15,68 Regulation of chondrocyte proliferation and hypertrophy, the main determinants of bone length growth, has not been completely elucidated. It has been suggested that growth hormone acts directly on stem cells in the resting zone and promotes their differentiation into daughter cells capable of producing insulin-like growth factor-1 (IGF-1). These cells, under autocrine or paracrine influence of IGF-1, stimulate clonal expansion of chondrocytes in the proliferative zone.101,143 Ample evidence indicates that the change from a proliferative chondrocyte phenotype to a hypertrophic chondrocyte phenotype is mainly controlled by a local feedback loop involving parathyroid hormone–related peptide, Indian hedgehog, and transforming growth factor-β (TGF-β). However, other regulators may also be involved.3,25,80,85 Endochondral ossification is the conversion of newly formed cartilage into metaphyseal bone. Chondrocytes in the zone of mineralization undergo their terminal differentiation and secrete and maintain a highly specialized matrix that promotes matrix calcification.3 Chondrocytes juxtaposed to the metaphyseal vasculature (terminal hypertrophic chondrocytes) die abruptly by apoptosis.37,38 Clasts remove the transverse matrix septa separating apoptotic hypertrophic chondrocytes from the metaphyseal vasculature, leaving void lacunae.84 Newly formed vasculature originating from the metaphysis and osteoprogenitor cells invade the vacated lacunae. Vascular endothelial growth factor (VEGF) produced by hypertrophic chondrocytes appears to control vascular ingrowth.56,121 Osteoprogenitor cells differentiate into osteoblasts, which lay down woven bone on the calcified cartilage matrix of the longitudinal septa, thus forming a lattice consisting of calcified cartilage and woven bone (primary spongiosa). Both calcified cartilage and woven bone are replaced by lamellar bone (secondary spongiosa), which completes the conversion from cartilage to metaphyseal bone. Regulation of the conversion of cartilage to woven bone is complex, but it appears that genetic, nutritional, metabolic, and mechanical factors all play a role.3,56,85,121 The cartilaginous ends of developing bones, the articular–epiphyseal complex, consist of two functionally different layers: a relatively thin outer layer and a relatively thick inner part (see Figure 73-1, C).74 The most superficial, outer layer consists of specialized immature articular cartilage, which is avascular and takes no part in the process of endochondral ossification. It will develop into mature articular cartilage consisting of a superficial zone, a transitional zone, a radial zone, and a zone of calcified cartilage.21,74 The uncalcified radial zone is separated from the zone of calcified cartilage by a microscopically visible line, the so-called tidemark (Figure 73-3).29,74,144 Emergence of the tidemark indicates completion of the maturation process. The inner layer is functionally similar to the growth plate and is responsible for epiphyseal enlargement by new cartilage formation and mineralization of newly formed cartilage. Morphologically, two main differences between this layer and the growth plate have been noted: (1) the inner layer is visually disorganized and does not have the ordered zonal and columnar arrangement of the growth plate, and (2) unlike the proliferative, hypertrophic, and mineralization zones in the growth plate, the chondro-epiphysis has an abundant vasculature.18 The chondro-epiphyseal blood vessels originate from the perichondral plexus and course within so-called cartilage canals (Figure 73-4, A). They are distinct from vessels on the metaphyseal side of the growth plate. In their terminal branches, afferent arterioles and returning venules form glomeruli.86,159,160 Anastomoses between cartilage canal vessels have not been demonstrated.153 It is thought that these cartilage canals play a role in nourishing epiphyseal chondrocytes, in forming and maintaining the epiphyseal ossification center, and in providing mesenchymal stem cells.165 Just as in the growth plate, enlargement of the epiphysis is a function of chondrocyte proliferation, chondrocyte enlargement, matrix formation, and duration of chondrogenic activity in the chondro-epiphysis. Most cell proliferation takes place on the periphery, whereas conversion of newly formed cartilage into bone occurs toward the center, where chondrocytes are larger and are surrounded by more matrix. As the ossification front from the center of the epiphysis reaches newly formed epiphyseal cartilage, the redundant cartilage canals regress through a normal process called chondrification.86 It has been demonstrated that blood vessels from the epiphyseal bone marrow anastomose with cartilage canal vessels.113,166 These anastomoses develop only toward the end of the growth period (Figure 73-5, A and B). Following completion of epiphyseal growth and ossification, a thin layer of avascular articular cartilage and a cartilage disc sandwiched between epiphyseal and metaphyseal bone (growth plate) remain. Regulation of epiphyseal enlargement and endochondral ossification is not as well understood as regulation of the growth plate. However, it most likely has strong similarities with regulation of the growth plate. Osteochondrosis is a condition seen in many animal species and human beings. It has a multifactorial origin with several suggested risk factors playing a role: heredity, rapid growth, dietary factors, and trauma.35,144,165 Strong predisposition in breeds and blood lines within breeds for the different forms of osteochondrosis strongly suggest a heritable etiologic component. In dogs, breed predispositions have been demonstrated for all forms of osteochondrosis, and reported heritability for these conditions ranges from 10% to 45%.10,82 It has been suggested that most forms of canine osteochondrosis are inherited as a polygenetic trait.10 In human beings and pigs, severity of osteochondrosis lesions may be associated with certain joint shapes and exterior conformations.52,54,55,135 In pigs, selection using criteria based on joint shape decreased the incidence of severe osteochondrosis lesions.51 Thus, anatomic features of affected joints may play an etiologic role in the development of osteochondrosis in certain species, and these anatomic factors, at least in part, may be genetically determined. In the literature addressing canine disease, joint morphology has been suggested as an etiologic factor for osteochondrosis. Elbow incongruity due to a relatively long ulna (ulnar overgrowth) following asynchronous growth of the radius/ulna is associated with a fragmented medial portion of the coronoid process. Similarly, elbow incongruity due to a relatively long radius (radial overgrowth) is associated with an ununited anconeal process.161 Another genetically determined morphologic factor may be the presence or absence of a growth plate of the anconeal process: only dog breeds that have this growth plate can develop ununited anconeal process. It has been reported that in dogs, rapid growth increases the incidence of skeletal diseases, including osteochondrosis.31,125,126 The predisposition of large- and giant-breed dogs with a rapid rate of skeletal growth would be consistent with this notion.82 It has been hypothesized that overfeeding energy (ad libitum feeding) will result in elevated levels of growth hormone, IGF-1, triiodothyronine (T3), thyroxine (T4), and insulin, subsequently stimulating chondrocyte differentiation and proliferation, and thus growth plate–related growth.125 It also has been hypothesized that the relatively increased growth rate from overnutrition predisposes the development of osteochondrosis lesions by increasing muscle mass and body weight, resulting in biomechanical overload of joint surfaces that are still structurally weak.31 This notion is based largely on studies in Great Dane puppies fed ad libitum or restricted amounts of a diet rich in protein, energy, calcium, and phosphorus.62 Dogs from the group fed ad libitum developed relative failure of cartilage maturation, but articular osteochondrosis was found in only 5 of 24 dogs: 3 on ad libitum and 2 on restricted feeding. In a recent review, a direct role of rapid growth from overnutrition in the origin of osteochondrosis was questioned.165 It has been suggested, mostly on the basis of a series of studies in Great Dane puppies,61,62,100,129,145 that a diet high in calcium and/or high in vitamin D3 may be associated with developmental orthopedic diseases such as osteochondrosis.50,59,62,133 From these studies, it is evident that such diets cause a disturbance in endochondral ossification, but a direct correlation with osteochondrosis has not been established. Furthermore, these studies were not recapitulated in other predisposed breeds, so it remains unknown whether these findings were unique to Great Danes, or whether they can be generalized to other dog breeds. Thus, although ample evidence indicates that diets high in calcium and/or high in vitamin D3 affect endochondral ossification in Great Dane puppies, no clear evidence suggests that high dietary calcium concentrations will cause or increase the risk for osteochondrosis of articular surfaces and growth plates of long bones in dogs from other breeds. The contention that macrotrauma is an etiologic factor in osteochondrosis is based largely on studies in human beings and pigs, and no studies about the relationship of macrotrauma and the development of osteochondrosis in dogs have been reported. In human beings, stress due to increased athletic activity appears to increase the prevalence and severity of macroscopic osteochondral lesions.7 Macrotrauma in pigs (dropping young pigs from varying heights) was associated with a greater number of osteochondrosis dissecans lesions seen at slaughter.99 Both studies reported on the chronic phase of osteochondrosis and not on the initiation of lesions. Thus, major trauma may hasten the conversion from an osteochondrosis manifesta lesion to an osteochondrosis dissecans lesion, but evidence that macrotrauma may play a role in the development of early lesions is lacking.165 It has been suggested on the basis of studies in pigs that microtrauma may be an important factor in lesion development and prevalence, and that microtrauma affecting cartilage canal vessels at the chondro-osseous junction may be the initiating event of osteochondrosis (see Figures 73-4, B and C and 73-5C).165,166 In dogs, exercise (playing one or more times per day with other dogs) has been mentioned as a risk factor for osteochondrosis.133 It also has been suggested that a relatively long ulna (ulnar overgrowth) may cause chronic overload, microtrauma of the medial coronoid process, and subsequent fragmentation of the medial portion of the coronoid process.161 However, the major loading area of the proximal humeral joint surface only partially coincides with the area where osteochondrosis lesions of the caudal humeral head develop.75 Thus, the role of microtrauma in articular osteochondrosis in the dog remains to be determined. Microtrauma may play a role in the development of ununited anconeal process: radial overgrowth may cause shear forces in the growth plate of the anconeal process, ultimately resulting in failure and ununited anconeal process.161 Theories about the pathogenesis of osteochondrosis of the articular–epiphyseal complex consider osteochondrosis as an expression of a generalized disease or as a manifestation of a focal disturbance. Two theories in which osteochondrosis is considered the result of a generalized disease have been proposed. In the first, it was hypothesized that the primary lesion in osteochondrosis is a dyschondroplasia, a generalized abnormality of chondrocyte development and maturation that prevents matrix mineralization and subsequent bone formation.107,108 It has been suggested that osteochondrosis lesions develop secondarily in response to biomechanical stress. This theory does not sit comfortably with the species-specific, site-specific, and often bilaterally symmetric morphology of osteochondrosis, although it is a prevailing theory in equine osteochondrosis.63,165 No reported morphologic evidence supports this hypothesis in dogs. It also has been hypothesized that relative osteopenia of subchondral bone, secondary to overnutrition and rapid growth in large- or giant-breed dogs, is unable to provide biomechanical support to the adjacent cartilage. Chondrocyte metabolism is consequently disrupted, leading to osteochondrosis.31 Just as with the previous theory, this hypothesis does not address site predispositions for the disease. Another limitation is that a relationship between relative osteopenia and initiation of the osteochondrosis lesion has not been demonstrated. This theory is not considered a primary pathomechanism of osteochondrosis.165 It also has been proposed that osteochondrosis starts as a focal disease resulting from vascular trauma and subsequent necrosis of subchondral bone or necrosis of epiphyseal cartilage canals. Necrosis of subchondral bone can occur through trauma, which initially leaves the overlying cartilage intact.127,151 The overlying cartilage may become secondarily affected, with osteochondrosis defects occurring several months later. These lesions have been documented with arthroscopy and magnetic resonance imaging (MRI).7 Evidence in support of this theory, described in the human medical literature, has not been found on histologic examination of early lesions in animals.35 On the basis of histologic studies in pigs and horses, it has been suggested that the primary lesion of osteochondrosis is a focal necrosis of epiphyseal cartilage canals, resulting in areas of cartilage ischemia and necrosis. This necrosis may occur at a development stage (age-dependent) when the vessels originating from the perichondrium are being replaced by vessels from adjacent epiphyseal bone marrow (see Figures 73-4 and 73-5). As vessels become incorporated into the ossification front, they presumably become susceptible to damage by local conformational forces and/or microtrauma. These vessels may become necrotic and nonperfused with a resultant cartilaginous infarct (Figure 73-6).20,22,144,166,167 The infarcted cartilage focally prevents endochondral ossification. The matrix within the necrotic cartilage degenerates following the death of chondrocytes.164 Chondrocytes in surrounding healthy growth cartilage increase matrix production and upregulate the production of VEGF, with adjacent cartilage canal vessels proliferating toward the lesions.164,166 Neighboring subchondral bone demonstrates an inflammatory reaction. This early lesion coincides with the designation osteochondrosis latens.167 The thickening cartilage may become radiographically visible and less resistant to mechanical stress and may be metabolically deprived and degenerate (termed osteochondrosis manifesta167). Although these lesions have been studied mainly in swine and equids, they have been seen in the femoral condyles of young dogs,35 but not cats. This theory is compelling, in that it is compatible with a disease process of growing animals with growth cartilage becoming avascular as the animal matures. It also may be consistent with the site-specific and often bilaterally symmetric nature of osteochondrosis, as the juvenile vascular supply has a consistent species-specific pattern and tends to demonstrate bilateral symmetry. Several sequelae have been proposed (see Figure 73-6). The necrotic focus may become organized and may heal through stages of granulation tissue that are converted to bone by intramembranous ossification.165 The weakened cartilage also may deform, resulting in altered joint contour and congruency.111 If necrotic cartilage persists, a subchondral bone cyst may occur.4,133 To our knowledge, this pathologic sequence has not been described in the cat. Alternatively, fissures, clefts, or cracks may start at the necrotic cartilage lesion. If the necrotic lesion is sufficiently large, these defects may propagate along the osteochondral junction or tidemark for some distance. If they extend to the joint surface, a cartilage flap is “dissecated” off the underlying subchondral bone. This results in typical signs of synovitis, joint effusion, arthralgia, lameness, and osteoarthritis typically accompanying this form of the disease. This is the most commonly recognized manifestation of osteochondrosis and generally is known as osteochondritis dissecans30,35,107,111,124 or osteochondrosis dissecans.165 This form of the disease has been described as a grade IV lesion; milder grades are rarely diagnosed and do not produce clinical signs (Table 73-3).144 Table • 73-3 Pathologic Grading of OCD Lesions in the Proximal Humerus30
Osteochondrosis
Definition and Classification
Epidemiology
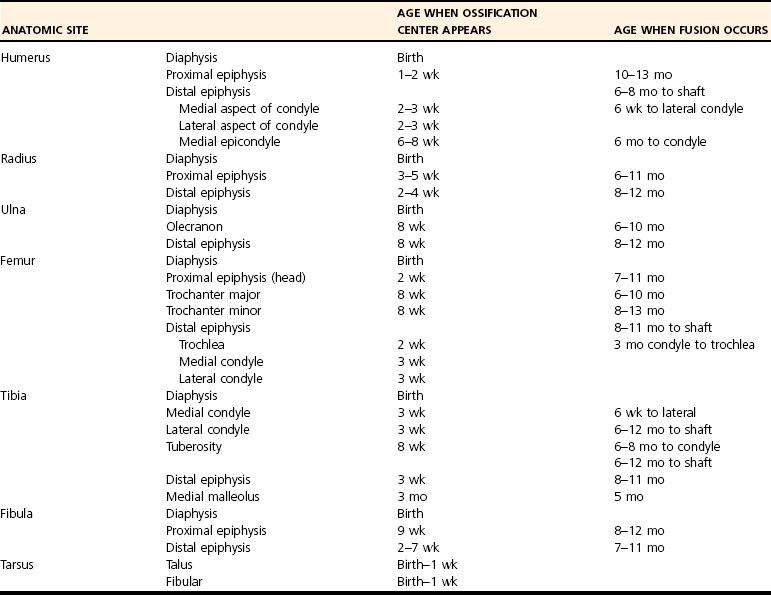
Skeletal Development
Growth Plate Enlargement and Endochondral Ossification
Epiphyseal Enlargement and Endochondral Ossification
Etiology and Risk Factors
Pathogenesis and Pathology
Articular–Epiphyseal Cartilage Complex
GRADE
DESCRIPTION
I
Cartilage surface normal; cartilage slightly thickened; “minuscule” subchondral defect
II
Cartilage surface mottled; cartilage more thickened; small cleft between cartilage and subchondral bone
III
Discoid elevation of cartilage surface; large cleft; underlying sclerotic subchondral bone
IV
Typical partially detached cartilage flap or separated flap and joint mice
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Osteochondrosis
Only gold members can continue reading. Log In or Register to continue