Chapter 54 Oropharynx
Structure and Function
Structure of the Oropharynx
Oral Cavity
The oral cavity extends from the lips to the oropharynx, or more specifically to level of the palatine tonsils.1 The lateral boundaries are the cheeks and the dorsal boundary is the hard palate and part of the soft palate.1,2 The ventral boundary is the tongue and floor of the mouth. The oral cavity encompasses the vestibule and the oral cavity proper. The vestibule is the potential space between the lips or cheeks and the teeth and gums. The oral cavity proper extends from the alveolar ridge and teeth to the oropharynx.1 The oral cavity proper contains the teeth, hard palate and part of the soft palate, portions of the osseous tissue of the skull and mandible, and the tongue.
Dogs and cats have anisognathic jaws as the mandible is narrower than the maxilla and the teeth of the maxilla and mandible do not meet directly in occlusion.1 The dentition and jaw structure in carnivores facilitate capture of prey and tearing of tissue versus the grinding and crushing action of the teeth and jaws in humans and other omnivores. The dentition of the dog is described as diphyodont, heterodont, and thecodont.1 Diphyodont means having two sets of teeth, such as a primary set that erupts soon after birth followed by a permanent set that remains in place for the remainder of life. Heterodont refers to having more than one shape of tooth, such as the pointed canines, which are used for grasping and holding food and the more blunt caudal molars that are used for grinding and crushing food. Thecodont teeth are held firmly within the sockets or alveoli by a type of fibrous, nonmobile joint termed a gomphosis.1–3 The fibrous structure that anchors the teeth to bone is more commonly referred to as the periodontal ligament. The alveolar processes of the paired maxillary and mandibular bones surround the roots of the teeth. It is composed of a cortical plate, cribriform plate, and trabecular bone. The cortical plate forms the outer wall of the alveolus. The cribriform plate is a thin layer of bone within the alveolus, which appears radiographically as the lamina dura. Trabecular bone is the supportive hard tissue between the cortical plate and the lamina dura.1
The hard and soft palates separate the oral and nasal cavities. The bony structure of the hard palate is composed of the paired incisive bones, palatine processes of the maxilla, and palatine bones. These bones are covered by cornified stratified squamous epithelial soft tissue, called the hard palate mucoperiosteum. The mucosa of the hard palate is nonelastic and has six to 10 transverse ridges or rugae. Located just caudal to the maxillary incisors on midline is a mound of tissue called the incisive papilla. On either side of the papilla are the incisive ducts, which travel through the palatine fissures of the incisive bones to communicate with the vomeronasal organ.1–5 The greater palatine branch of the maxillary branch of the trigeminal nerve (cranial nerve [CN] V) supplies sensory innervation to the hard palate. The main arterial supply is from the paired greater palatine arteries that branch off from the maxillary arteries.2,4 The soft palate begins at the caudal termination of the hard palate and continues into the pharyngeal region; it is discussed further with the oropharynx.
An important structure within the oral cavity is the tongue, which is responsible for prehension and manipulation of a food bolus during mastication and deglutition. It is also involved in grooming and intake of fluids. The tongue is divided into four sections: tip, margin, body, and root. It becomes thicker caudally toward the root. Arterial blood supply to the tongue is provided by the paired lingual arteries.2 Motor innervation of the tongue is from the hypoglossus. Sensory innervation is provided by the lingual branch of the trigeminal (CN V), facial (CN VII), glossopharyngeal (CN IX), and vagus nerves (CN X).2,4 The dorsal surface of the tongue is covered by cornified lingual papillae. The papillae can be mechanical or may contain taste pores which provide information to the sensory nerves.2 Five types of papillae are present in the tongue of the adult dog: filiform, fungiform, vallate, foliate, and conical. A sixth type, called marginal papillae, is a functional type present only in neonates. The marginal papillae are located on the margins of the tongue and help create an airtight seal in the mouth during nursing. They begin to disappear soon after weaning. Filiform papillae are numerous and are located on the rostral two-thirds of the tongue. They are heavily cornified and may aid in grooming; thus they are mostly mechanical in function. The filiform papillae in the cat are especially stiff and long, and are oriented in a caudal direction.4 The fungiform papillae are also located on the rostral two-thirds of the tongue but are mostly concentrated on the tip and sides of the tongue. Each fungiform papilla may contain up to eight taste pores. There are anywhere from three to six total vallate papillae in the dog. They are arranged in a V shape at the base of the tongue. Vallate papillae may also contain taste pores. The foliate papillae do contain taste buds and are located on the caudal third of the tongue immediately rostral to the palatoglossal folds. Finally, the conical papillae are located on the caudal third of the tongue. Their function is mainly mechanical and tactile and they do not contain taste pores.1,2 A visible median groove divides the most rostral two-thirds of the dorsal tongue. The ventral surface is covered by a smoother, less cornified mucosa.1 A band of tissue called the lingual frenulum extends from the floor of the mouth to the base of the tongue.
The tongue is able to prehend and manipulate food as a result of its composition of intrinsic and extrinsic skeletal muscles. The intrinsic propria lingua muscle is innervated by the hypoglossal nerve (CN XII) and serves to retract and depress the tongue. It has four types of muscle fibers: superficial longitudinal, deep longitudinal, perpendicular, and transverse. These muscle fibers allow the tongue to perform complex movements during prehension, fluid intake, mastication, bolus formation, and deglutition and also function to prevent the tongue from being bitten. The extrinsic muscles are the styloglossus, hyoglossus, and genioglossus. These extrinsic muscles are also innervated primarily by the hypoglossal nerve (CN XII). The origin of the styloglossus muscle is the stylohyoid bone. The styloglossus muscle has three heads and the tongue can be pulled backward when all three heads are contracting together.2 Each head can also act to depress the tongue. The hyoglossus muscle originates from the basihyoid and thyrohyoid bones to the root and caudal two-thirds of the tongue. Its action is to depress and retract the tongue. The genioglossus muscle lies beneath the tongue in the intermandibular space and its origin is on the medial surface of the mandibles. A portion of the genioglossus makes up the lingual frenulum. The caudal fibers can act to draw the tongue forward and the rostral fibers can curl the tip of the tongue downward.2
Other important structures involved in the function of the oropharynx are the salivary glands. These accessory digestive organs secrete a serous and mucous fluid (saliva) that is important for lubrication of a food bolus for transport to the upper digestive tract. Many different salivary glands contribute to saliva formation and they are regulated by the autonomic nervous system (see Chapter 1). There are several groups of numerous, small, disseminated glands that secrete small amounts of saliva. These are the lingual, labial, buccal, and palatine salivary glands. The larger salivary glands contribute to the bulk of saliva formation and are the paired parotid, mandibular, sublingual, zygomatic, and, in cats only, the molar glands.1 The parotid gland is located close to the surface of the masseter muscle. The parotid duct exits into the vestibule on the buccal mucosa at the level of the maxillary fourth premolar. The zygomatic salivary gland, which is located ventral to the rostral portion of the zygomatic arch, has a duct that exits in the vestibule just caudal to the parotid duct. There may also be several small minor zygomatic duct openings in addition to the major opening.1–4 The parotid duct termination is commonly called the major papillae and the larger zygomatic duct opening is called the minor papillae. The mandibular gland is located just caudal to the angle of the mandible between the linguofacial and maxillary veins. The sublingual gland is divided into monostomatic and polystomatic portions. The monostomatic portion of the gland is located primarily within the capsule of the mandibular salivary gland with a few lobules of tissue located close to the mandibular and sublingual ducts near the root of the tongue. The polystomatic portion is a group of small, scattered lobules that empty through several minor sublingual ducts into the oral cavity between the tongue and mandible.1 The mandibular and sublingual salivary glands are intimately associated with one another as they share a common capsule. Their ducts are adjacent to one another and terminate in the same location. The openings of the ducts of the mandibular and the monostomatic portion of the sublingual gland are located beneath the tongue in a fold of mucosal tissue called the sublingual caruncle. Salivary mucoceles are most commonly caused by a defect in the sublingual gland or duct. Because of the close proximity of the mandibular and sublingual glands, treatment for salivary mucoceles requires excision of the mandibular and sublingual gland ducts to the level of the lingual nerve.6–8 Buccal and lingual molar glands are present only in the cat. The buccal molar gland is located below the mucous membrane of the lower lip and empties into the vestibule through several small ducts. The lingual molar gland is located just lingual to the mandibular first molar in a fold of tissue. This gland also has several small openings unlike a common duct.1
Oropharynx
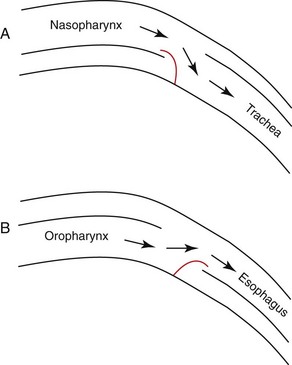
The pharynx is the common passageway for the respiratory and digestive systems. It provides a connection between the nasal and oral cavities to the trachea and esophagus and is pivotal in directing air, food, and fluids into the correct system. The pharynx encompasses the nasopharynx, oropharynx, and laryngopharynx. The nasopharynx is part of the respiratory system. It is located dorsal to the soft palate and extends from the choanae to the caudal free edge of the soft palate and the palatopharyngeal arches.3 This caudal border is where the laryngopharynx begins. The oropharynx is part of the digestive system and lies between the soft palate, the base of the tongue, and the epiglottis. The laryngopharynx receives air from the nasopharynx and directs it to the trachea, and accepts food and water from the oropharynx and directs it to the esophagus. It is a part of both the respiratory and digestive systems and extends from the base of the epiglottis to the esophagus.3
The anatomic borders of the oropharynx are indistinct but can be correlated with certain structures. The dorsal border of the oropharynx is the ventral surface of the soft palate. The soft palate is the muscular continuation of the hard palate and is covered by stratified squamous epithelium on the ventral surface. Dorsal to the epithelium there are numerous palatine glands, which open on the ventral surface of the soft palate. The next layer is the muscular layer and includes the paired palatine muscles and the end portions of the paired tensor and levator veli palatini muscles. The arterial vascular supply of the soft palate includes the minor palatine arteries, the ascending pharyngeal artery, and the major palatine arteries. Venous drainage occurs through the palatine plexus.2 The right and left pterygopharyngeal muscles originate from the pterygoid bones and pass laterally and dorsally along the pharynx and the caudal part of the soft palate to form the base for the palatopharyngeal arches. These arches are the caudal continuation of the soft palate and also demarcate the nasopharynx from the laryngopharynx.2,4 The paired palatoglossal arches or folds are located at the rostral border of the soft palate and demarcate the end of the oral cavity and the beginning of the lateral borders of the oropharynx. These arches are not well-defined structures compared to humans because dogs and cats lack a palatoglossus muscle. When the tongue is pulled rostrally and laterally the fold is more visible and extends from the body of the tongue to the rostral border of the soft palate.2 Soft palate defects are caused by disturbances during development of the secondary palate. These defects can occur on midline between the palatine muscles, or less commonly can be unilateral or bilateral and are located lateral to the palatine muscles. In bilateral defects of the soft palate, a remnant of tissue remains that is composed of portions of the levator and tensor palatini muscles, connective tissue, and mucosa.9 In contrast to an abnormal or absent soft palate, brachycephalic breeds commonly have an elongated soft palate, which can interfere with the function of the glottis and epiglottis or the normal passage of air.2,10
The lateral walls of the oropharynx are termed the fauces. The fauces contain the palatine tonsils that reside within the tonsillar fossa. The tonsils are aggregates of lymphoid tissue located in the pharyngeal mucosa. They are not encapsulated like typical lymph nodes. The tonsils function as host defense at the mucosal level. Only efferent lymphatic drainage occurs as tonsillar tissue does not filter lymph.2,4 The palatine tonsils are the only discrete bodies of lymphoid tissue. The other tonsils such as the pharyngeal and lingual are more diffuse. The pharyngeal tonsil is unpaired and is known as the adenoid in humans. The lingual tonsils are distributed over the entire surface of the base of the tongue.11 The palatine tonsils arise from the second branchial cleft epithelium. The palatine tonsils often increase in size over time as an increase in lymphatic tissue and the system of crypts occurs, likely as a consequence of antigenic stimulation.2,11 These crypts serve as physical antigen traps and the tonsils themselves form immunocompetent lymphocytes.8 The palatine tonsils can be visible in some animals or they may be completely within the tonsillar fossa. Everted tonsils are more often seen in brachycephalic breeds, perhaps as a result of increased negative airway pressure causing eversion of the tonsils and laryngeal saccules.10 The medial wall of the fossa is formed by the tonsillar fold that comes from the ventral surface of the lateral portion of the soft palate.2 The deep portion of the tonsil is attached to the lateral wall of the pharynx. Efferent drainage of the palatine tonsils is into the medial retropharyngeal lymph node. Blood supply is derived from the tonsillar artery, which is a branch of the lingual artery. The palatine plexus provides venous drainage.2
Function of the Oropharynx
Prehension and Mastication
The most important functions of the oral cavity and oropharynx are their roles in prehension, mastication, bolus formation, and deglutition or swallowing. Prehension is the grasping and manipulation of food. In dogs and cats, prehension is generally achieved with the use of the tongue, teeth, and mandible. The lips play a very minor role in prehension and act more to keep food and fluids in the oral cavity. The olfactory nerve (CN I), optic nerve (CN II), trigeminal nerve (CN V), hypoglossal nerve (CN XII), and the cerebral cortex control the voluntary act of prehension.12,13 Vertebrates with complete cheeks, such as pigs, sheep, and horses, use suction to draw liquid upward and use their tongue to transport it intraorally. In contrast, vertebrates with incomplete cheeks, including most carnivores, are unable to seal their mouth cavity to generate suction and must instead rely on their tongue to move water into the mouth. The cat accomplishes this through a rapid lapping mechanism that differs in function from the scooping mechanism of the dog.14
Mastication is the process of breaking down food into smaller pieces and coating it with saliva to prepare a food bolus for deglutition. The masticatory muscle group is composed of the paired temporalis muscles, masseter muscles, lateral and medial pterygoid muscles, and the digastricus muscles. The masseter, pterygoids, and temporalis muscles contain special type 2M skeletal muscle fibers, sometimes referred to as superfast fibers.4,15 Masticatory muscle myositis, a disease characterized by painful inflammation, scarring, and contracture of the masticatory muscles, can be diagnosed by measuring serum 2M antibody. Autoantibodies against this specific type of myosin in these fibers are responsible for the immune-mediated process.16 An increase in titer along with clinical signs confirms the diagnosis.17 The muscles of mastication are innervated by the mandibular branch of the trigeminal nerve (CN V) except for the caudal belly of the digastricus muscle, which is innervated by the facial nerve (CN VII).2 All of the muscles, except for the digastricus, act to close the jaws. The digastricus muscle contracts to open the jaws. The large size of the masseter and temporalis muscles and the action of the pterygoid muscles act to keep the jaws closed even at rest. Prehension, mastication, and bolus formation are voluntary whereas deglutition is strictly involuntary.
Deglutition
Deglutition is the transport of a bolus of food or liquid from the mouth to the stomach.12 Normal deglutition requires precisely timed contraction and relaxation of numerous muscles of the oral and pharyngeal regions (Table 54-1).18 After prehension and mastication the food bolus stimulates sensory receptors in the oropharynx that inhibit the muscles of mastication and allows swallowing to occur. The structures involved in deglutition include the tongue, hard and soft palate, pharyngeal muscles, esophagus, and gastroesophageal junction.19 Coordination of swallowing is controlled by the trigeminal (CN V), facial (CN VII), glossopharyngeal (CN IX), vagus (X), and hypoglossal (CN XII) nerves and their nuclei. These nerves and nuclei are themselves controlled by areas of the reticular formation known as the swallowing center.20 All of these nerves provide sensory and motor innervation except for the hypoglossal (CN XII), which provides only motor innervation.21 The trigeminal nerve (CN V) carries sensory fibers from the oral cavity and motor fibers for the muscles of mastication and the soft palate muscles. The facial nerve (CN VII) contains sensory fibers from visceral receptors in the soft palate and nasopharynx, and motor fibers to the stylohyoid and omohyoid muscles. The glossopharyngeal nerve (CN IX) contains sensory fibers to the pharynx and some areas of the tongue. The vagus nerve (CN X) and the motor fibers of the glossopharyngeal nerve (CN IX) innervate nearly all of the muscles of the pharynx. The vagus nerve carries both visceral efferent and visceral afferent fibers to the pharynx. The visceral sensory nerves of the vagus nerve transmit impulses from the base of the tongue, pharynx, and esophagus to the medulla oblongata.19
Table 54-1 Muscles and Motor Nerves Involved in Prehension, Mastication, and Deglutition
| Muscle | Innervation | Action |
|---|---|---|
| Lingua propria, intrinsic tongue muscle (P, D) | Hypoglossus | Protrude the tongue, coordinate complex movements, prevent tongue from being bitten |
| Hyoglossus, extrinsic tongue muscle (P, D) | Hypoglossus | Retract and depress the tongue |
| Styloglossus, extrinsic tongue muscle (P, D) | Hypoglossus | Draw the tongue backward or depress the tongue |
| Genioglossus, extrinsic tongue muscle (P, D) | Hypoglossus | Depress the tongue, draw the tongue forward, curl the tip downward |
| Temporalis muscle (P, M) | Mandibular branch of trigeminal nerve | Closing the jaws |
| Masseter muscle (P, M) | Mandibular branch of trigeminal nerve | Closing the jaws |
| Medial and lateral pterygoid muscles (P, M) | Mandibular branch of trigeminal nerve | Closing the jaws |
| Digastricus muscle—rostral belly (P, M) | Mandibular branch of trigeminal nerve | Opening the jaws |
| Digastricus muscle—caudal belly (P, M) | Facial | Opening the jaws |
| Hyopharyngeus (D) | Glossopharyngeal and vagus | Constriction of the rostral pharynx |
| Thyropharyngeus (D) | Glossopharyngeal and vagus | Constriction of the middle part of the pharynx |
| Cricopharyngeus (D) | Glossopharyngeal and vagus | Constriction of the caudal part of the pharynx |
| Stylopharyngeus (D) | Glossopharyngeal and vagus | Dilation, elevation, and drawing of the pharynx forward |
| Palatopharyngeus (D) | Glossopharyngeal and vagus | Constriction and drawing of the pharynx forward and upward |
| Pterygopharyngeus (D) | Glossopharyngeal and vagus | Constriction and drawing of the pharynx forward |
| Tensor veli palatini (D) | Mandibular branch of the trigeminal | Stretching of the soft palate |
| Levator veli palatini (D) | Glossopharyngeal and vagus | Raising of the caudal part of the soft palate |
| Palatinus (D) | Glossopharyngeal and vagus | Shortening and curling of the posterior border of the soft palate |
D, swallowing function; M, masticatory function; P, prehensile function.
Deglutition is divided into three phases: oropharyngeal, esophageal, and gastroesophageal. The oropharyngeal phase is controlled by the trigeminal (CN V), facial (CN VII), glossopharyngeal (CN IX), vagus (CN X), and hypoglossal nerves (CN XII). The oropharyngeal phase is further subdivided into three stages: oral, pharyngeal, and pharyngoesophageal.12,13,19 The oral phase is voluntary and is controlled by cranial nerves V, VII, and XII. The food bolus is transferred into the oropharynx by the base of the tongue.
Once the food bolus reaches the pharynx, the involuntary pharyngeal phase begins. Sensory receptors in the oropharynx are stimulated and transmitted by cranial nerves V, IX, and X. The swallowing center in the medulla oblongata initiates the deglutition reflex and causes progressive contraction of the pharyngeal muscles to continue to propel the food bolus. To prevent aspiration the soft palate is elevated to seal off the nasopharynx and the entrance to the trachea is covered by the glottis and epiglottis (Figure 54-1). The pharyngeal phase is controlled by cranial nerves V, VII, IX, X, and XII. The final stage of oropharyngeal deglutition is the pharyngoesophageal phase. Cranial nerves IX, X, and the swallowing center control this stage. The cricopharyngeus and thyropharyngeus muscles of the cranial esophageal sphincter relax to allow the food bolus to pass into the cranial portion of the esophagus. The sphincter closes after passage of the bolus to aid in retention and to prevent aerophagia. A primary peristaltic wave in the esophagus is initiated after passage of the bolus (see Chapter 55).12,13,19,20
Inflammation
Stomatitis
Clinical Manifestations
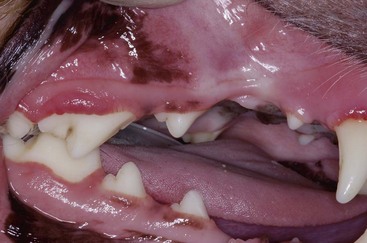
The major clinical sign often is halitosis. Depending on the severity of stomatitis, there may be hypersalivation, drooling, decreased food intake, dysphagia, lethargy, depression, and weight loss (Figure 54-2). Patients with extensive inflammation and ulcers are often in pain and are apprehensive about being touched around their head or mouth. Oral examination should be attempted with these patients; however, when the lesions are too painful, this is done under anesthesia unless anesthesia is contraindicated.
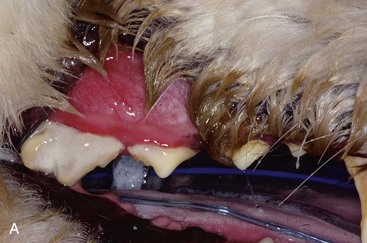
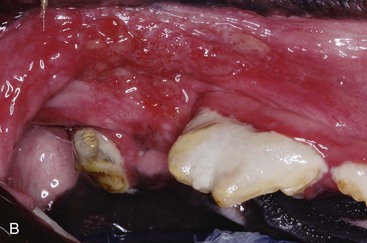
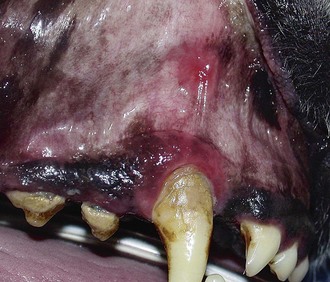
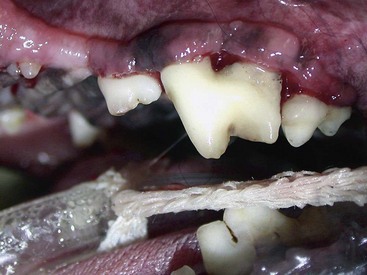
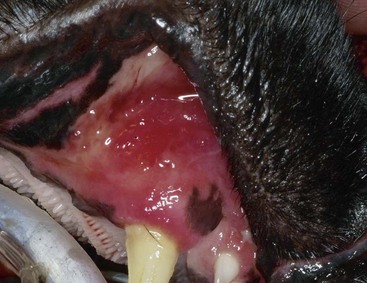
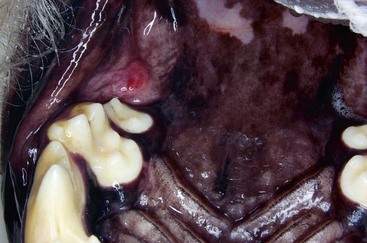
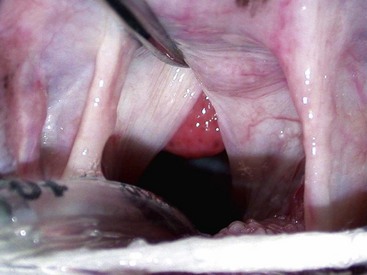
Gingivitis is inflammation of the gingiva and may be limited to the marginal gingiva or include the attached gingiva (Figure 54-3). Focal areas of gingival hyperplasia may result from chronic inflammation. Contact ulcers are focal areas of inflammation and ulceration located on the buccal mucosa where it contacts plaque biofilm (Figure 54-4). Periodontitis involves the gingiva as well as the other tissues of the periodontium (i.e., cementum, periodontal ligament, alveolar bone). Oral examination may reveal gingival recession, gingival hyperplasia, root exposure, and mobile teeth (Figure 54-5).
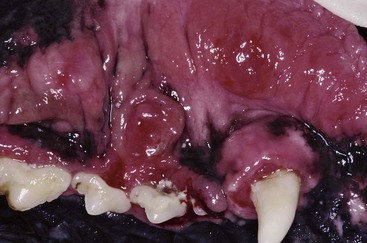
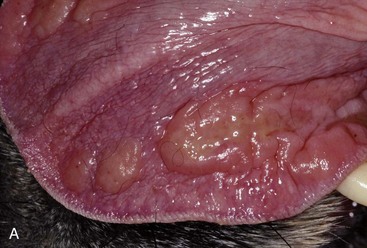
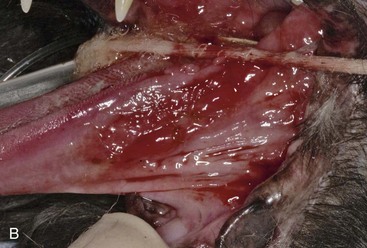
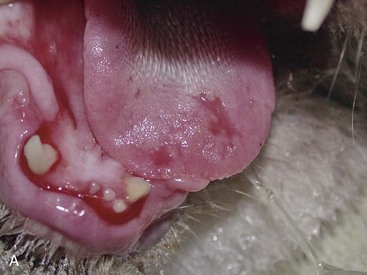
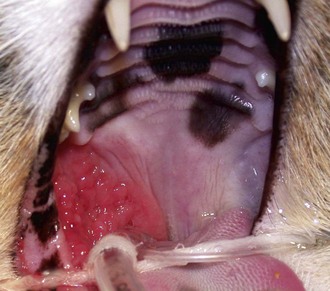
Patients with stomatitis may have severe inflammation and ulceration of the gingiva, buccal mucosa, tongue, and palate (Figure 54-6). Inflammation of the tongue is often along the lateral margins and there may be ulceration are severe cases (Figure 54-7). Palatal inflammation and ulceration is usually linear or focal and range from superficial to deep ulceration. Patients with severe stomatitis may be in significant pain. Clinical signs may be severe halitosis, hypersalivation, drooling, and changes in behavior. The patients often refuse dry food and will eat only soft food. In very severe cases they refuse soft food as well. When cats with caudal stomatitis open their mouths to eat or yawn this can trigger intense pain, and they may vocalize, aggressively rub or scratch at their face, run away from their food, and have activities that the owners describe as a seizure. On physical examination there may be minimal external signs. In severe cases there is blood-tinged or thick-yellowish saliva, and saliva may adhere to the fur around the lips and on the front legs. Cats often have a matted and unkempt appearance. The inflammation of the mucosa is often disproportionately severe compared with the minimal amount of plaque and calculus on the teeth.
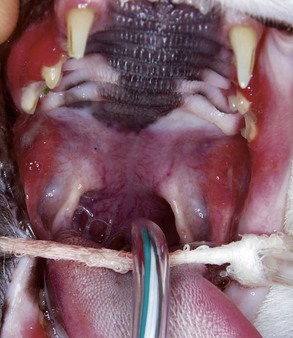
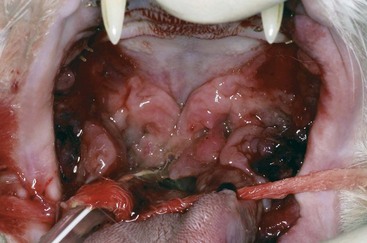
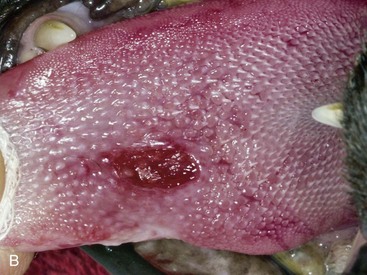
The inflammation in cats with caudal stomatitis initially may be limited to the pterygomandibular raphe (Figure 54-8). Over time many cats with caudal stomatitis will also develop anterior stomatitis (Figure 54-9). Pharyngeal hyperplasia, in severe cases, can result in a narrowing of the pharyngeal opening, contributing to pain on swallowing (Figure 54-10). Oral examination of cats with severe caudal stomatitis may be difficult, but visualization is often possible if the mouth is opened very slowly and carefully. Inflammation of the tongue may be local or generalized and present as erythematous areas, erosions, or ulcers. Cats with lingual ulceration may have clinical manifestations of upper respiratory tract infection. Lingual ulcers may be located anywhere but the most common areas are the rostral one-third of the tongue (Figure 54-11). Lingual ulcers associated with feline calicivirus (FCV) and feline herpesvirus (FHV)-1 are seen with the acute phase of the infection and when there is a recurrence of signs in a carrier cat.
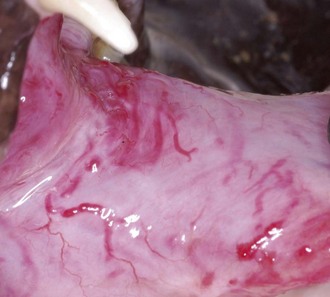
Sublingual masses generally occur at the base of the tongue with varying degrees of inflammation, ulceration, and proliferation. When a lingual mass is present, especially if the base of the tongue is involved, the patient may have difficulty eating or drinking (Figure 54-12).
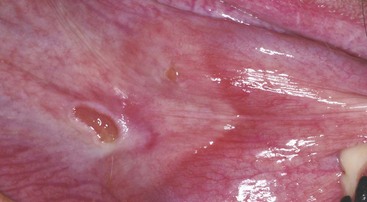
Eosinophilic diseases affecting the oral cavity occur more frequently in cats than in dogs.1 The canine breed reported most commonly with eosinophilic granulomas is the Siberian Husky. Affected dogs have lesions primarily on the palate or tongue.2,3 Eosinophilic granulomas have also been reported in Cavalier King Charles Spaniels.4–6 Clinical signs may not be present and the lesions may be found incidentally on physical examination. Lesions on the palate or tongue are usually linear with raised margins, and a reddish color in the center but no ulceration (Figure 54-13). Eosinophilic granulomas in cats occur primarily at the base of the tongue, are proliferative with varying degrees of ulceration, and may have a similar appearance to sublingual squamous cell carcinoma. Clinical signs may include excessive licking and abnormal tongue movement with difficultly in eating or drinking.
Oral candidiasis may result in single or multiple erosions or ulcers of the mucocutaneous junctions and oral mucosa. Ulcers are nonhealing and erythematous, and are covered by whitish-gray plaques.2,7
Lymphoma in the oral cavity may be seen as raised, inflamed gingival tissues, or as a mass lesion.8
Patients with systemic diseases may have oral lesions as part of the presenting clinical signs. Vasculitis as a consequence of systemic disease may lead to oral lesions including ulcers and necrosis of oral mucous membranes and the tongue. Additional oral lesions that may be associated with systemic diseases include congestion, hemorrhage, glossitis, uremia-related oral ulceration, and lingual necrosis, especially at the tip of the tongue.2
Pathogenesis
Inflammation of the oral tissues is a common pathologic response when disruption of the normal oral mucosal barrier allows an inflammatory response in the epithelium or connective tissues.9
The masticatory mucosa of the gingiva, hard palate, and the dorsal surface of the tongue is a tough layer of keratinized stratified squamous epithelium that provides protection from compressive and shear forces.10 This protective layer is less likely to be damaged with resulting inflammation compared with other areas in the oral cavity.
The lining of the oral mucosa is composed of nonkeratinized stratified squamous epithelium that is attached to underlying structures by loose, elastic connective tissue. The tightly adherent epithelial cells and intercellular ground substance compose the primary mucosal barrier and protect the underlying tissues against fluid loss and the ingress of potentially harmful environmental agents (e.g., microbial toxins and antigens). This permeability barrier also isolates organisms in the epithelial layer from larger molecules (e.g., antibodies, complement) in the subepithelial tissues. When antigens penetrate the mucosal barrier, antigen–antibody complexes, complement activation, and infiltration of inflammatory cells result in tissue destruction. Oral organisms on the surface of the epithelium do not penetrate the mucosal barrier unless the organism acquires a greater virulence or the host defenses are compromised.10
The protective function of the mucosal barrier may be damaged by trauma, immune-mediated disease, soluble toxins, altered mucosal barrier, and xerostomia.10 When the mucosal layer is damaged or there is a decrease in cell renewal, epithelial and subepithelial inflammation may occur. Xerostomia and dehydration may result in ulceration and infection because of the role of salivary mucins in maintaining healthy epithelium.
Epithelial cell renewal is affected by age, stress, inflammation, chemotherapy, and radiation treatments. Mild epithelial inflammation stimulates cellular proliferation while severe inflammation suppresses cell proliferation.10 Chemotherapeutic agents and radiation treatments limit the proliferative ability of the epithelium and connective tissue. Depending on the neoplastic disease and planned cancer treatment, the oral cavity should be evaluated and oral disease (e.g., dental) managed prior to treatment to prevent complications associated with mucositis.11
The pathogenesis of periodontal disease depends on microbial plaque stimulating an initial inflammatory response. The host defense cells are stimulated to produce and release mediators capable of destroying the alveolar bone and periodontal connective tissue. Genetics plays a role in determining the host inflammatory response.12,13
Patients infected with Cryptococcus neoformans occasionally have oral involvement as part of the clinical manifestations of local or systemic disease. The lesions may include diffuse ulceration of the buccal mucosa, tongue, gingiva, hard palate, and lips as well as gingival proliferation. The more common clinical signs of cryptococcosis include sneezing, snuffling, nasal discharge, skin lesions, and a nasal mass.8,14,15
Vasculitis as a manifestation of systemic or local disease affects the circulation and oxygenation of tissues and may lead to destruction or necrosis of these tissues.16
Etiology and Differential Diagnosis
Stomatitis is defined as the inflammation of soft tissues of the oral cavity occurring as a result of mechanical, chemical, thermal, bacterial, viral, electrical, or radiation injury, or as a reaction to allergens or as a secondary manifestation of systemic disease.17
Caudal stomatitis in cats is secondary to altered immune function; however, the etiology has not been determined.18 Various theories have been presented, including associations with FCV and Bartonella henselae.19 FCV does appear to be associated with caudal stomatitis.19–21 The role of FHV-1 in caudal stomatitis is unknown; however, in one study the majority of cats with caudal stomatitis were shedding both viruses.21
Eosinophilic diseases in most cases are attributable to hypersensitivity reactions to insects (e.g., fleas and mosquitoes), environmental antigens (e.g., atopic dermatitis, foods), or other antigenic stimulation. Eosinophilic granulomas in some cats may be a result of imbedded insect parts; in many cases, however, the specific etiology remains unknown.1 Pemphigus vulgaris, bullous pemphigoid, and pemphigus foliaceus may have oral manifestations. Of these, pemphigus vulgaris is most likely to have oral manifestations and up to 75% to 90% of dogs with this disease have involvement of the oral cavity. Oral lesions are the initial clinical signs in 50% of cases.2
Painful oral ulceration and inflammation may occur following radiation treatment or chemotherapy.
Extension of diseases such as neoplasia (e.g., squamous cell carcinoma) and infection into the oral cavity can occur, resulting in inflammatory lesions within the oral cavity.22
Systemic diseases also may have oral manifestations. Renal failure and vasculitis (e.g., rickettsial diseases) from any cause may result in oral manifestations (Figure 54-14).16 Mucosal erosion and ulceration may be present in patients with systemic lupus erythematosus. Bacterial (e.g., Francisella tularensis, Leptospira canicola), fungal (e.g., C. neoformans), and viral infections, neoplasia, and drug reactions all have the potential for oral manifestations.8,23,24
Diagnosis
Caudal stomatitis in cats is a clinical diagnosis and is present only when there is inflammation of the pterygomandibular raphe. This is important because there are specific treatments for caudal stomatitis that do not apply to stomatitis elsewhere in the mouth. Histologic examination reveals inflammation and does not add to the diagnosis; however, when neoplasia is suspected based on the clinical appearance, histological examination should be undertaken (see Figure 54-14).
Eosinophilic granuloma (complex) is diagnosed by histopathology (rules out other problems); the underlying etiology, however, may require additional evaluation (e.g., dietary trials).1
Histopathology is necessary for the diagnosis of pemphigus vulgaris, oral candidiasis, and neoplasia.7
Treatment
Pain management is especially important initially when there is severe inflammation and ulceration. Oral antimicrobial rinses (e.g., 0.12% chlorhexidine gluconate) are often used until the epithelium has healed. Secondary bacterial infections may occur in cases that do not rapidly resolve and systemic antibiotics may be necessary in addition to the topical rinses.25
Periodontal disease treatment planning is based on the extent of disease. Complete periodontal treatment planning is beyond the scope of this chapter.13
Eosinophilic granuloma complex may be treated with short courses of glucocorticoids if the lesions are mild and occur only once to twice a year.1 If lesions are progressive in severity or frequency, a more complete evaluation for the cause should be undertaken. Higher doses and longer durations of glucocorticoids, antibiotics, and cyclosporin may be used in the more severe cases.1
Treatment of caudal stomatitis has been widely debated over the years; however, the first treatment should be extraction of all premolar and molar teeth. In some patients with severe anterior stomatitis accompanying the caudal stomatitis, extraction of the canine and incisor teeth may also be necessary. Medical treatment following surgical extractions may include antibiotics, glucocorticoids, or cyclosporin. Medical management should not be used in place of extractions for long-term management. Clindamycin, amoxicillin-clavulanate acid (Clavamox), and metronidazole are commonly used oral antibiotics. If glucocorticoids are used as an adjunct to extraction, they should be used at the lowest doses necessary. Cyclosporin has been used for the management of cats with persistent clinical signs following extractions. Cats should be treated for at least 8 weeks to assess the clinical response.26 Potential side effects of cyclosporin are gingival hyperplasia, excessive shedding, and hypertrichosis.27,28 The immunosuppressive effects of cyclosporin may result in development of infections or the emergence of an existing subclinical infectious disease (e.g., toxoplasmosis).29–31 Although lactoferrin, gold salts, and other miscellaneous treatments have been recommended, there is no evidence that they have any clinical benefit. Newer therapies that have been proposed are omega interferon (Virbagen Omega, Virbac) and T-cell receptor (TCR) peptides (TCR Vax). Anecdotally, cats with an incomplete response to extractions have improved with Virbagen Omega; however, this product is not licensed in the United States. The administration of a TCR vaccine for treatment of caudal stomatitis in cats has been proposed (www.imulan.com). TCR Vax is undergoing field trials and is not yet commercially available.
Pemphigus vulgaris is difficult to treat and multidrug therapy is often necessary. High-dose glucocorticoid administration either alone or in combination with another immunomodulatory medication is usually recommended. Azathioprine is the secondary immunomodulatory medication most commonly used.32 Gold salts, chlorambucil, and tetracycline-niacinamide are other options for the treatment of pemphigus. Alternative therapeutics may be tried when the response to the commonly used drugs is not adequate. Intravenous immunoglobulin (IVIg) has been suggested as an alternative therapeutic for treating dogs.32 IVIg used in humans for the treatment of pemphigus has shown beneficial results and a better quality of life when compared with other therapeutics.33
Mucositis secondary to chemotherapy or radiation treatment is managed with palliative care. Pain medications, topical rinses (e.g., CET rinse), and soft foods are used during the healing phase. Infections (e.g., candidiasis) and systemic complications may occur during treatment or after treatment.25
Prognosis
The prognosis for resolution of clinical signs in cats with caudal stomatitis is dependent on the treatment, severity of disease, concurrent viral (e.g., FCV) infection, and duration of disease. An excellent response following extraction may be achieved, especially when treated at an early stage. Extraction of the premolars and molars carries the best long-term prognosis for resolution.34 The prognosis is poor to guarded when extractions are not done and when tooth roots have not been removed. Cats that do not have a successful or significant improvement in clinical signs following extraction are medically managed. There is a varied response in these cats and some may have excellent responses. In one study, cyclosporin treatment resulted in remission in four of eight cats and fair to good improvement in the other cats.26
Eosinophilic granuloma, especially in dogs, may spontaneously regress. Eosinophilic granulomas in cats may respond well to three monthly subcutaneous injections of a long-lasting glucocorticoid. Cyclosporin for treatment and maintenance is reported to be beneficial.26
Radiation mucositis is an acute reaction and should start to heal by 10 to 14 days after treatment has ceased. However, the prognosis depends on the amount of radiation that the tissue was exposed to. The prognosis is guarded in patients with severe mucositis and poor healing.25 Postradiation osteonecrosis has a poor prognosis and a comprehensive assessment and oral care should be done before radiation therapy.35
Pharyngitis
Clinical Manifestations
The pharynx is divided into three areas: nasopharynx, oropharynx, and laryngopharynx. Clinical manifestations vary depending upon the location and extent of the inflammation, the etiology, and the involvement of adjacent structures. The most common clinical manifestations include dysphonia (voice change), snoring, coughing, gagging, dysphagia, inappetence, and hypersalivation. When stomatitis occurs concomitantly with pharyngitis there may be clinical signs of each. Secondary tonsillitis is often present in association with pharyngitis. Clinical manifestations of tonsillitis are similar to those of pharyngitis (Figure 54-15).
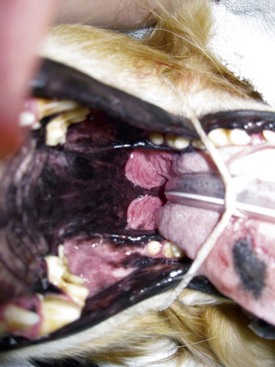
Figure 54-15 Bilateral tonsillar enlargement with histologic evidence of chronic inflammation and fibrosis.
Nasopharyngeal foreign bodies can cause coughing, sneezing, epistaxis, gagging, discomfort on pharyngeal palpation, and open-mouth breathing.36 Obstructive masses may result in dyspnea, stridor, open-mouth breathing, snoring, and gagging.36–38
Disease involving extrapharyngeal sites have additional clinical manifestations and signs of disease (e.g., neurologic signs, ocular discharge, repeated head shaking) depending on the involved site, disease process, and extent of disease.38
Etiology and Differential Diagnosis
Pharyngeal inflammation can occur from trauma, caustic or irritating substances, infectious diseases, obstructive masses, foreign bodies and as an extension of rhinitis, sinusitis, caudal stomatitis, and drainage of a local abscess (e.g., retrobulbar abscess) (Figure 54-16).36,39 Caustic or irritating substances are similar to those causing stomatitis. Pharyngitis caused by chronic coughing, regurgitation, or vomiting may cause irritation resulting in pharyngitis. Severe cases of caudal stomatitis in cats can extend into the oropharynx. Sino-orbital aspergillosis may extend into the palate and nasopharynx resulting in clinical signs of pharyngitis.21,40,41
Nasopharyngeal diseases include lymphoma and inflammatory polyps.40,41 Feline inflammatory polyps that expand into the nasopharynx as well as other obstructive masses in the oral cavity or nose, may obstruct the flow of air and the exaggerated respiratory efforts result in laryngeal inflammation (Figure 54-17).38 The etiology of feline inflammatory polyps has not been determined.38 Tissue from 41 inflammatory polyps was evaluated by polymerase chain reaction for the presence of FCV and FHV-1; negative findings suggested that these viruses are not involved with the pathogenesis of inflammatory polyps.38
Treatment
Topical irritants, depending on the source, should be removed as much as possible by rinsing the mouth. Foreign bodies are removed using direct visualization.36
Nasopharyngeal inflammatory polyps in cats with normal bulla may be treated (removed) by traction avulsion.38 A novel approach to removal of polyps using an endoscope passed into the nasopharynx via a gastrotomy has been described.42
Nasopharyngeal stenosis in cats may be treated by balloon dilation under general anesthesia.43,44 Restenosis may occur and it may be treated with another balloon dilation. When retreatment is necessary, the application of topical steroids or an antifibrotic agents may be tried to decrease the degree of another restenosis.43
Sialadenitis
Pathogenesis
The pathogenesis of salivary gland inflammation may be idiopathic, infectious (e.g., bacterial, viral, mycotic), traumatic, secondary to xerostomia or dehydration, ductal obstruction, or organic disease of the gland (e.g., Sjögren syndrome). Acute bacterial infections of the salivary gland are usually a result of an ascending infection. Xerostomia or dehydration may contribute to the development of ascending bacterial infection by decreased salivary flow and alteration of the normal oral flora.45,46 Hematogenous spread of infection from other areas also may cause a bacterial sialoadenitis.47
Etiology and Differential Diagnosis
Ascending bacterial infections are most likely to be caused by anaerobic periodontal pathogens.48 Decreased salivary gland flow may occur secondary to dehydration, salivary duct obstruction, or decreased saliva production. Acute salivary gland inflammation or chronic inflammation with fibrosis may decrease salivary gland production. Systemic disorders that may lead to inflammation include autoimmune sialadenitis (e.g., Sjögren syndrome) and vasculitis.49 Sialadenitis is uncommon in dogs and cats. Differentials for enlarged salivary glands include sialadenitis, sialadenosis, and infiltrative disease.
Diagnosis
Diagnostic tests may include fine-needle aspirate cytology, culture and sensitivity, histopathology, ultrasound, and radiography (e.g., films and contrast sialography). Fine-needle aspirate cytology of enlarged salivary glands is the initial diagnostic test and may reveal the presence of salivary gland inflammation.50 Bacterial culture and sensitivity of exudate from the orifice of the salivary duct or the salivary gland is recommended.47 Ultrasound of the salivary gland can help to differentiate obstructive from nonobtrusive sialadenitis and identify masses and abscess formation.51 Radiography, including plain films and contrast sialography, may identify radiopaque salivary calculi and ductal or glandular changes. Histopathology may reveal neutrophils in the ducts and small abscesses in the interstitium.47
Treatment
Sialadenitis is treated with supportive care, antiinflammatories, and antibiotics.52 Abscesses may be treated by antibiotics in addition to drainage using ultrasound guidance.51
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree