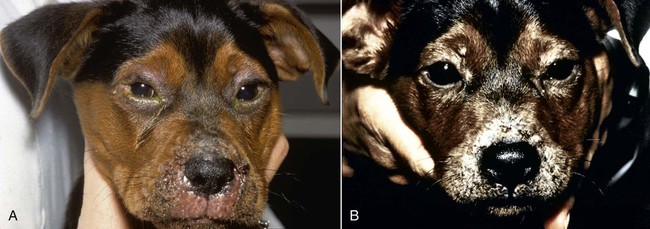
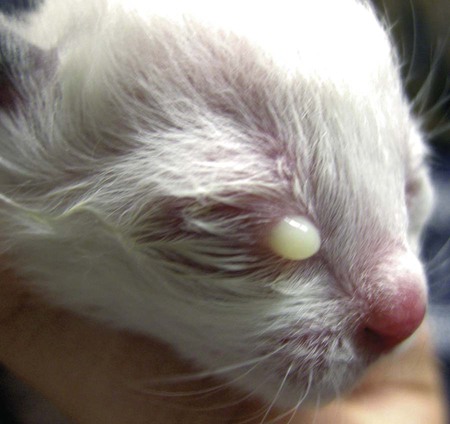
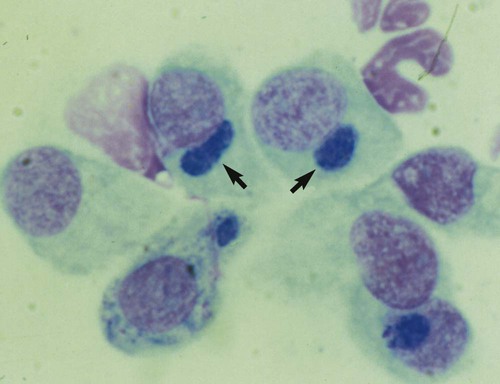
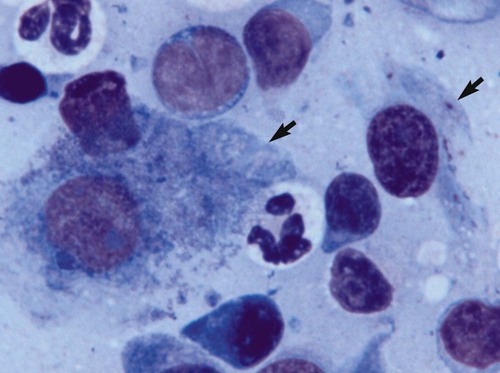
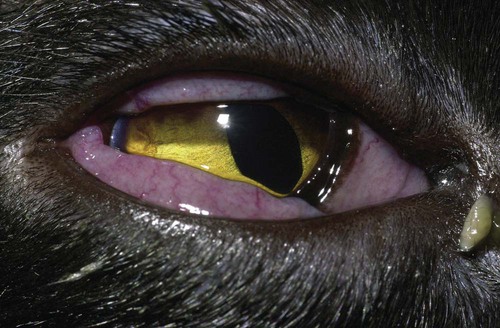
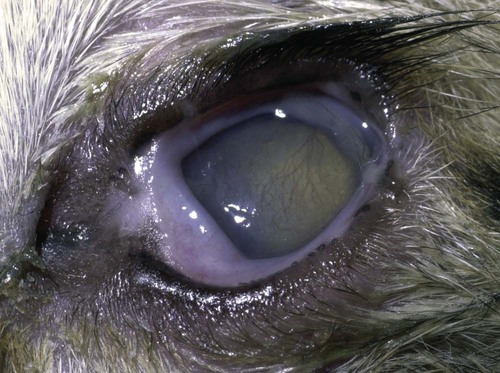
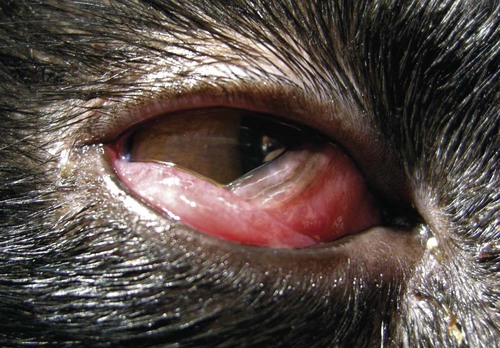
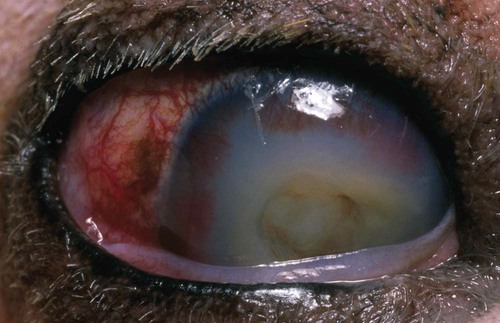
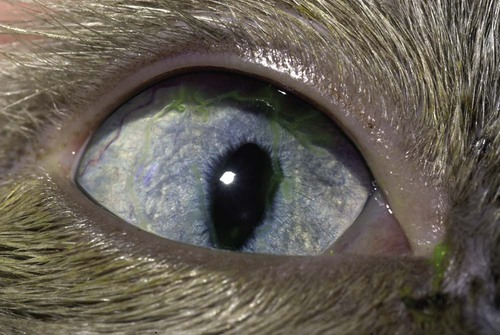
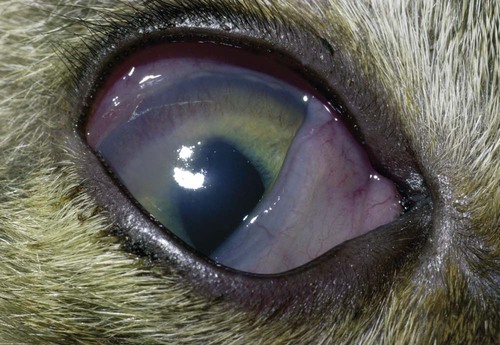
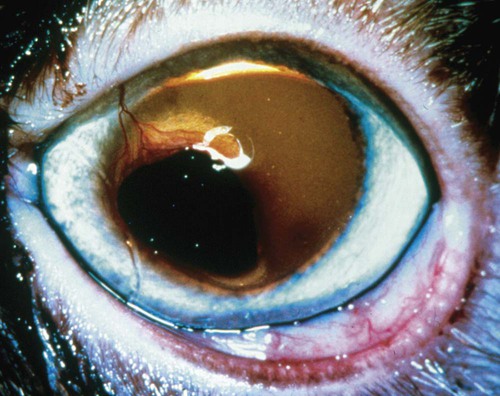
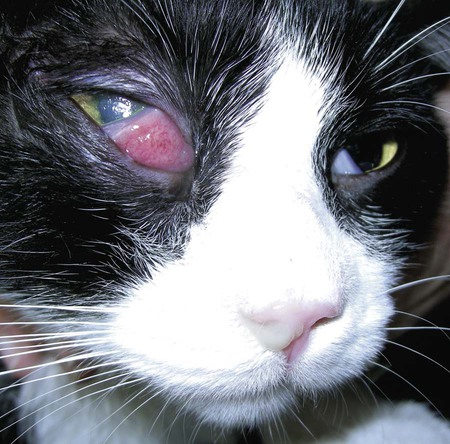
Despite the fact that all dogs probably have indigenous bacteria in their conjunctival cul-de-sac, positive isolation rates of between 46% and 91% in clinically healthy dogs have been reported (Table 92-1). Variations in the type of and frequency of isolates may be a result of geography, culturing technique, breed, and season. Fungi are isolated from the conjunctival sac in 10% to 22% of dogs.59,137 TABLE 92-1 Frequency of Bacterial Isolation from the Conjunctival Sacs of Clinically Healthy Dogs10,58,69,165 aPercentages are based on the numbers of animals from which organisms were isolated. In contrast to dogs, cats have a relatively lower rate of cultivable bacteria in their conjunctival sacs.45 Bacteria or mycoplasmas have been isolated from 34% of the conjunctival samples and from 25% of the samples from the lid margins. In one study of 50 cats (100 eyes), no organisms were isolated from 42% of the cats, bacteria were isolated from the conjunctival sacs of 34% of the cats or 47% of the eyes, and 26% of the cats or 14% of the eyes had fungal isolates from the conjunctiva (Table 92-2). No anaerobes were isolated.57 TABLE 92-2 Bacterial and Fungal Isolates from Clinically Healthy Cats45,57 aPercentages are based on the numbers of animals from which organisms were isolated. Most surface bacterial infections are not strictly primary; other debilitating conditions often potentiate the pathogenicity of organisms that are indigenous to the ocular surface. Other local nidi of infection, such as in the nasolacrimal (NL) system and meibomian glands or structures adjacent to the eye (ears, lip folds), should be sought and corrected to overcome persistent or recurring infection. Control of the resident ocular flora of clinically healthy animals is maintained by rinsing of the ocular surface with tears and blinking, which pushes the tears into the NL system. Tears also contain IgA and other antibacterial substances such as lactoferrin. Competitive interaction among the indigenous flora keeps the numbers of organisms lower, whereas disrupting this balance may cause an overgrowth of one species. Conditions debilitating to the ocular surface, such as keratoconjunctivitis sicca (KCS), ultraviolet or other radiation, immune suppression, and trauma creating breaks in the corneal or conjunctival epithelial barrier, may allow indigenous bacteria to adhere and possibly overgrow to produce disease. To become established, bacteria must adhere, replicate, and then invade the tissue. Invasion subsequently incites inflammation. Tissue damage with infection produces a combination of toxins from the microorganism and enzymes such as collagenase, elastase, and cathepsins liberated by the neutrophilic response.141 Bacteria, such as Pseudomonas aeruginosa, that produce proteolytic enzymes typically cause rapidly progressive corneal ulcers. Canine juvenile pyoderma or cellulitis develops occasionally in pups ages 3 to 16 weeks (Fig. 92-1). A hypersensitivity reaction to canine distemper vaccines (see Chapters 3 and 100) or Staphylococcus species has been suggested; in one study, Staphylococcus was isolated from draining lesions in only 2 of 15 puppies.169 Although the cause is unknown, the response to treatment suggests it to be immune-mediated. Pustules and pyogranulomatous inflammation develop in the eyelids, face, and ears. Conjunctivitis is usually present. Mandibular lymphadenomegaly is common. The pups may be febrile and have generalized malaise. Swelling of joints or metaphyses may also be observed. Canine herpesvirus (CHV) was documented as a cause of severe conjunctivitis and ulcerative keratitis in a group of 3- to 4-month-old laboratory dogs.95 The dogs had recovered from upper respiratory tract disease that was not characterized. In a study of naïve adult dogs experimentally infected with CHV via an ocular route, only self-limiting mild conjunctivitis was noted.91 Similarly, in a controlled study of client-owned dogs, with and without naturally acquired idiopathic conjunctivitis, CHV or canine adenovirus-2 were found in 23.3% of affected dogs and 0% of clinically healthy dogs.92 PCR methods have facilitated detection of CHV in affected animals.35,93 If CHV is suspected or documented as a cause of conjunctivitis, a topical antiviral agent such as trifluridine or idoxuridine should be used (Table 92-3). See also Chapter 5. Topically applied prednisolone acetate solution (1%) did not reactivate latent herpes infection93; however, systemic administration of prednisolone at immunosuppressive doses resulted in reactivation of ocular illness and shedding of virus.94 TABLE 92-3 Commercially Available and Compounded Antiherpetic Agents aDose based on in vitro studies of feline herpesvirus (FHV)-1 and reports from human medicine. bSee the Drug Formulary in the Appendix for further information. Neonatal conjunctivitis is an acute inflammation of the conjunctiva that can occur secondary to bacterial or viral pathogens. If infection occurs before the opening of the eyelids, at approximately 10 days of age, purulent material builds up, causing a characteristic distended appearance to the adherent eyelids. In such cases, the eyelids should be opened by inserting a small scissor blade at the medial canthus and gently sliding it along the lid margins. Copious purulent discharge will result (Fig. 92-2). Both bacterial and viral cultures, as well as cytologic examination of exudate, may be helpful in identifying the pathogens. A broad-spectrum topical antibacterial should be used several times daily. A topical antiherpesvirus agent should also be used in cases suspected to be caused by feline herpesvirus-1 (FHV-1) (see Chapter 2 and Table 92-3). Bacterial conjunctivitis in nonneonatal cats is unusual, with the exception of infection with Chlamydophila felis, which is a common cause of conjunctivitis in young cats (see Chapter 28). The clinical appearance is indistinguishable from conjunctivitis caused by FHV-1, and the two organisms may be present simultaneously. Diagnosis of Chlamydophila infection is based on seeing the reticulate bodies in the cytoplasm of conjunctival epithelial cells (Fig. 92-3) or obtaining a positive PCR or direct fluorescent antibody (FA) test result on a conjunctival swab or scraping. Cp. felis can also be cultured but requires special transport media and culture conditions. Reticulate bodies are often few in number and the numbers diminish with chronicity, making them easily missed. In an experimental study of chlamydial conjunctivitis, co-infection with feline immunodeficiency virus (FIV) prolonged the duration of clinical signs and led to chronic conjunctivitis.121 Conjunctivitis caused by Chlamydophila can be treated with topical tetracycline ointment applied to both eyes four times daily until 1 week past resolution. Oral doxycycline may be used instead or in addition to topical tetracycline and may be preferred to clear the organism from extraocular tissues such as the intestinal tract, reproductive tract, and visceral organs. Results of one study indicated that oral doxycycline at 10 mg/kg/day for at least 28 days was necessary to ensure elimination of the organism from tissues.34 The potential of this organism to infect people is questionable (see Public Health Considerations, Chapter 28); however, washing hands after treating an affected cat is advised. Hartmannella vermiformis, an amebic endosymbiont that contains the Chlamydia-like agent Neochlamydia hartmannellae, has been isolated in greater prevalence from the ocular surfaces of cats with keratitis or conjunctivitis than from those of clinically healthy cats.168 However, in another study, the identification rate of the organism between similar groups of cats was not different.134 The clinical significance of this organism is unknown (see Hartmannella Infection, Chapter 78). Mycoplasma felis has been variably implicated as a cause of conjunctivitis in cats (Fig. 92-4). Some studies have recovered Mycoplasma species as resident microflora from feline conjunctiva, whereas others have not. Similarly, experimental infections of clinically healthy, young cats have produced conjunctivitis in some studies68 and not in others. Mycoplasma organisms may require a stressor such as co-infection with FHV-1 to cause disease. One study reported a slightly higher percentage of cats with conjunctivitis to have higher positive PCR results for Mycoplasma spp. DNA (9.6%) as compared to FHV-1 (6.7%) or Cp. felis (3.2%) DNA.101 Mycoplasma species are sensitive to many topical antibacterials, including tetracyclines. FHV-1 is a frequent cause of ocular disease in cats.2,146,147,167 Young cats with respiratory tract disease generally have conjunctivitis with marked conjunctival hyperemia, chemosis, and serous to purulent ocular discharge (Fig. 92-5). Cats typically recover in 2 to 3 weeks. In severe cases of FHV-1 conjunctivitis, the risk of symblepharon, or adhesions of the conjunctiva to itself or the cornea, is high (Fig. 92-6). Symblepharon occurs when areas of conjunctival and corneal epithelial erosion are present as a result of the cytopathic nature of FHV-1. Symblepharon may lead to permanent visual impairment, and an attempt to break these adhesions early should be made. After application of topical anesthesia, a cotton-tip swab or small forceps can be used to break adhesions and strip off cellular debris and fibrin. This process may need to be repeated frequently until the conjunctivitis has resolved. In moderate or severe conjunctivitis, a topical ophthalmic antiherpetic agent should be used (see Table 92-3). In addition to general supportive care, the eyes should be cleansed frequently and a broad-spectrum topical antibacterial applied to minimize secondary bacterial infection as the conjunctival surface sloughs. Once infected, cats become latent carriers of FHV-1 and may have recurrences of ocular disease, including conjunctivitis and corneal ulcers. Many cats have transient episodes of conjunctivitis, with conjunctival hyperemia, serous or purulent ocular discharge, and blepharospasm. Mild cases that are self-limiting may not require treatment. If the conjunctivitis is painful or lasts more than a few days, antiviral therapy should be instituted. If a topical antibacterial is used, tetracycline is the most appropriate choice because of its efficacy in treating Cp. felis and M. felis, which are common feline conjunctival pathogens that may contribute to conjunctivitis in addition to FHV-1. Cats can develop chronic conjunctivitis associated with FHV-1.23,144 Laboratory diagnostic test results, such as those for virus isolation from the conjunctiva and conjunctival scrapings for direct fluorescent antibody testing, to prove FHV-1 as a cause of conjunctivitis in cats are frequently negative, making the diagnosis uncertain.119 Identification of FHV-1 DNA in conjunctiva or the cornea by PCR is a more sensitive test than virus isolation or direct FA testing, although clinically healthy cats may have positive test results for viral DNA by PCR.149,162 However, negative results on any of the foregoing tests do not rule out FHV-1 as the underlying cause of disease. Treatment with an ophthalmic antiviral medication can be used in cats with chronic conjunctivitis, although results vary. With chronic conjunctivitis, the inflammation generally needs to be treated. Glucocorticoids should not be used because of the potential to exacerbate an underlying FHV-1 infection. Topical nonsteroidal agents and 0.2% ciclosporin ointment (three to four times daily), used concurrently with an antiviral drug, appear to be both effective and safe. Recombinant interferons (IFN) have been administered to cats with FHV-1-related ocular disease, although no studies have documented effectiveness. IFNs-α and -ω have significant inhibitory effects on FHV in vitro at relatively high doses (see Chapter 2).138,140 Cats with experimental ocular infection that were pretreated, although not treated during clinical disease, with topical feline IFN-ω had no beneficial change in their clinical illness.70 High doses of topical IFNs have been used with some success in human herpetic infections.109,153,153 Treatment with oral lysine (see Table 92-3) has been shown to be efficacious in reducing the severity of FHV-1-induced conjunctivitis in an experimental setting.64,152 Cats receiving 500 mg of oral L-lysine twice daily had less severe conjunctivitis than cats receiving placebo, although the length of disease and isolation of virus did not differ between the groups (see Chapter 2). The use of oral famciclovir for treating FHV-1 in cats has become more common. The drug appears to be safe at least for a short course (2 to 3 weeks), although the optimum dose remains unclear, and safety in kittens has not been determined. Cats appear to absorb the drug from the gastrointestinal tract far less than do humans, dogs, and rats.160 A preliminary study indicated that a dose of 90 mg/kg every 8 hours was required to approach therapeutic plasma levels in the cat,159 although anecdotally many clinicians report beneficial effects with much lower doses (see Table 92-3). Feline calicivirus (FCV) is also a common cause of conjunctivitis in cats, particularly those in shelter or multi-cat environments. Conjunctivitis caused by FCV is indistinguishable from that caused by FHV-1 and may be very severe in some cats (Fig. 92-7) (see Chapter 14). The differentiation must be made by identifying the pathogen from virus isolation, PCR testing or direct FA staining results of conjunctival scrapings. Because FCV is an RNA virus, the ophthalmic agents available for treating FHV-1, a DNA virus, are ineffective. Treatment with a broad-spectrum topical antibacterial is appropriate, as are general supportive therapies. The role of FCV in causing chronic conjunctivitis in cats is unknown. Bacterial keratitis usually incites a visible neutrophilic response, characterized by a yellow to white cellular infiltrate within the cornea (Fig. 92-8). Vascular growth into the cornea also occurs but usually does not begin for several days after injury and infection. Blood vessels originate from the limbus and grow at a rate of approximately 1 mm/day toward the site of infection. Rapid corneal destruction can occur with infectious keratitis. Invading bacteria and neutrophils can release proteases and collagenases, which contribute to corneal melting. Increased cyclo-oxygenase-2 expression has been documented in all corneal layers of dogs with keratitis, indicating that antiprostaglandin therapy may be a potential treatment for inflammatory keratitis.139 Fungal keratitis, which can appear clinically similar to bacterial keratitis, is less common but can be caused by penetrating injuries with plant material or prolonged topical treatment with antibacterial or glucocorticoid therapy. Both bacterial and fungal pathogens may damage the cornea sufficiently to cause corneal perforation. Diagnosis of infectious keratitis is based on clinical findings, cytology, and culture. Fluorescein staining is used to detect epithelial ulceration. All corneal ulcers that are progressive or involve the stroma should be cultured. For specimen collection, a mini-tip Culturette with a sterile saline-moistened tip should be gently swabbed over the ulcer bed. After application of topical proparacaine, a flattened spatula, the blunt end of a scalpel blade, or a cytology brush should be used to gently scrape the ulcer edge for a cytologic sample. With bacterial keratitis, neutrophils are the predominant cell type, and bacteria can be numerous, few, or not visualized by cytologic examination. The initial decision about therapy should be based on whether cocci or rods have been identified. Culture is more sensitive than cytology for detecting bacteria and is the only way to confirm the species type and antibacterial susceptibility. Until culture results are known, the broadest spectrum of antibacterial drugs possible should be used. Often, this means combination therapy, such as a topical quinolone and cefazolin. To achieve broad-spectrum bactericidal activity, commercial antibacterials can be fortified by addition of additional drugs, and injectable antibacterials can be used with artificial tears or saline to create topical formulations (Tables 92-4 and 92-5). Frequency of application is critical. In a rapidly progressive corneal ulcer, topical antibacterials should be applied every 1 to 2 hours. TABLE 92-4 Commercially Available Ophthalmic Antibacterial Agents NA, Not available. TABLE 92-5 Fortified and Compounded Topical Antibacterial Solution Preparations Adapted from Ref. 170. If fungal keratitis is documented or suspected, it should be treated with application of a topical antifungal agent every 2 to 3 hours (Table 92-6). Although natamycin is the only approved ophthalmic antifungal agent in the United States, other agents such as silver sulfadiazine have proven effective and safe for topical ocular use.115 Compounding of other systemic or topical antifungal preparations for ocular use has been done by pharmacies. Topical miconazole or voriconazole solutions have been effective in treatment of keratomycosis in dogs.9,65 Deep corneal ulcers in danger of perforating respond best to surgical therapy, such as placement of a conjunctival flap. TABLE 92-6 aOnly commercially available ophthalmic antifungal agent in the United States. bNot available commercially for ophthalmic use. Must use existing parenteral solutions or have made by compounding pharmacy. cCommercially available as a dermatologic cream. Modified from Ref. 170. Bacterial keratitis in cats may develop secondary to corneal ulcers (including those initiated by FHV-1) or traumatic wounding. Appearance, diagnosis, and treatment are the same as for the dog. Although uncommon, fungal keratitis in cats can be treated similarly to that of the dog.81 Corneal ulceration and keratitis from FHV-1 is very common and begin with invasion of the corneal epithelium by the virus.146 The most common corneal abnormality is punctate or linear (dendritic) epithelial erosions (Fig. 92-9), which may quickly enlarge to form geographic superficial ulcers. Conjunctivitis usually accompanies corneal ulcers.144 Mechanical debridement of any loose corneal epithelium with a cotton-tipped swab and treatment with a topical antiviral agent have been the most successful therapies. A topical antibacterial should also be used for bacterial prophylaxis. Grid keratotomies should not be performed in cats because this treatment modality allows the virus greater access to the corneal stroma and appears to increase the risk of corneal sequestration.82 A topical antiherpetic agent should be used until the corneal ulcer has healed and for at least 1 week afterward (see Table 92-3). Clinically healthy cats without apparent infection can harbor the virus in a latent or an active form in their corneas or trigeminal ganglia.150,162 Experimentally, subconjunctivally administered dexamethasone caused cats infected with FHV-1 to develop stromal keratitis.116 Stromal keratitis can develop with or without a corneal ulcer. Corneal vascularization and cellular infiltrate, often accompanied by chronic discomfort, are typical symptoms (Fig. 92-10). Antiviral agents alone may not improve the keratitis. Topical anti-inflammatory agents may be needed, although glucocorticoids should be avoided. Nonsteroidal topical agents, and/or 0.2% ciclosporin ointment, can be used in conjunction with an antiviral agent. Topical glucocorticoids predispose cats to the development of corneal ulceration and sequestration when FHV-1 is present. Topical IFN has been used in humans with herpetic keratitis and may be beneficial in cats,109,153,153 although controlled studies are lacking (see Table 92-3). Oral lysine and famciclovir for ocular herpetic disease have been discussed under Conjunctivitis in Cats. Corneal sequestration is a common disorder in cats, particularly Persians and Himalayans, and can follow chronic corneal ulcers or keratitis caused by FHV-1 in any breed of cat (Fig. 92-11). It has occurred after topical glucocorticoid treatment in FHV-1 experimentally infected cats, and in cats receiving grid keratotomies.82,117 The condition is characterized by an area of corneal degeneration with a brown to black discoloration. The lesions vary from pinpoint sequestra to those that occupy more than half the cornea; vascularization may be intense or absent; and ocular pain ranges from none to marked. Like stromal keratitis, sequestra can be one of the most serious and potentially blinding sequelae of FHV-1. Most ophthalmologists recommend keratectomy followed by a graft (corneal or conjunctival) as the therapy for sequestra. Orbital disease caused by bacterial infection generally has a fairly rapid onset compared with the slow progression of clinical signs seen with orbital neoplasia. Typical clinical signs of orbital abscess and cellulitis include periorbital swelling, exophthalmos, elevation of the nictitating membrane, conjunctival hyperemia, and pain on palpation of periorbita or when opening the mouth (Fig. 92-12). Swelling of the oral mucosa behind the last molar is evident in some animals.
Ocular Infections
Extraocular Infections
Resident Microflora
Organism
Isolation (percentage)a
Staphylococcus (Total)
57–70
Coagulase-positive
24–45
Coagulase-negative
46–55
Streptococcus (Total)
6–43
Nonhemolytic
12–51
α-Hemolytic
4–34
β-Hemolytic
2–7
Corynebacterium (Total)
30–75
Undifferentiated
11
C. pseudodiphtheriticum
9
C. xerosis
13
Neisseria (Total)
26
Undifferentiated
4
N. catarrhalis
9
N. pharyngis
4
N. sicca
3
N. caviae
3
N. lactamica
3
N. flavescens
3
Pseudomonas (Total)
14
Moraxella (Total)
7
Bacillus (Total)
6–18
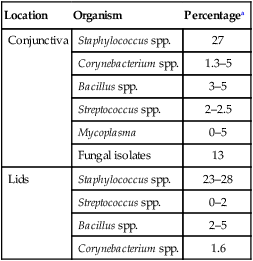
Location
Organism
Percentagea
Conjunctiva
Staphylococcus spp.
27
Corynebacterium spp.
1.3–5
Bacillus spp.
3–5
Streptococcus spp.
2–2.5
Mycoplasma
0–5
Fungal isolates
13
Lids
Staphylococcus spp.
23–28
Streptococcus spp.
0–2
Bacillus spp.
2–5
Corynebacterium spp.
1.6
Ocular Surface and Adnexa
Blepharitis in Dogs
Conjunctivitis in Dogs
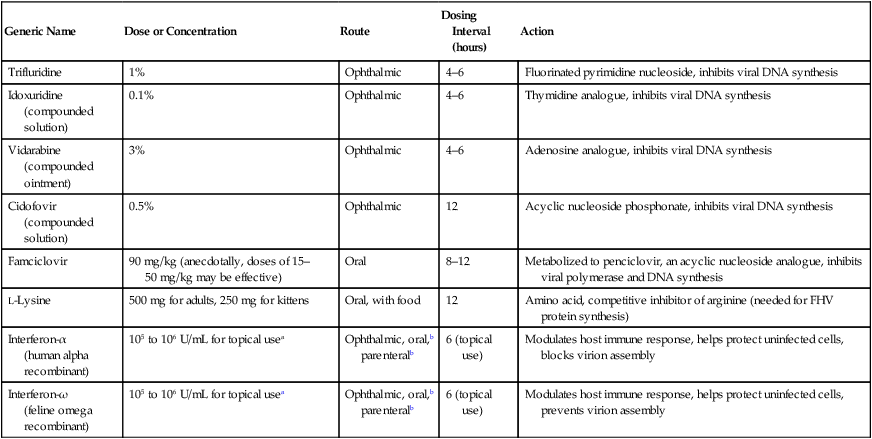
Generic Name
Dose or Concentration
Route
Dosing Interval (hours)
Action
Trifluridine
1%
Ophthalmic
4–6
Fluorinated pyrimidine nucleoside, inhibits viral DNA synthesis
Idoxuridine (compounded solution)
0.1%
Ophthalmic
4–6
Thymidine analogue, inhibits viral DNA synthesis
Vidarabine (compounded ointment)
3%
Ophthalmic
4–6
Adenosine analogue, inhibits viral DNA synthesis
Cidofovir (compounded solution)
0.5%
Ophthalmic
12
Acyclic nucleoside phosphonate, inhibits viral DNA synthesis
Famciclovir
90 mg/kg (anecdotally, doses of 15–50 mg/kg may be effective)
Oral
8–12
Metabolized to penciclovir, an acyclic nucleoside analogue, inhibits viral polymerase and DNA synthesis
L-Lysine
500 mg for adults, 250 mg for kittens
Oral, with food
12
Amino acid, competitive inhibitor of arginine (needed for FHV protein synthesis)
Interferon-α
(human alpha recombinant)
105 to 106 U/mL for topical usea
Ophthalmic, oral,b parenteralb
6 (topical use)
Modulates host immune response, helps protect uninfected cells, blocks virion assembly
Interferon-ω
(feline omega recombinant)
105 to 106 U/mL for topical usea
Ophthalmic, oral,b parenteralb
6 (topical use)
Modulates host immune response, helps protect uninfected cells, prevents virion assembly
Conjunctivitis in Cats
Keratitis in Dogs
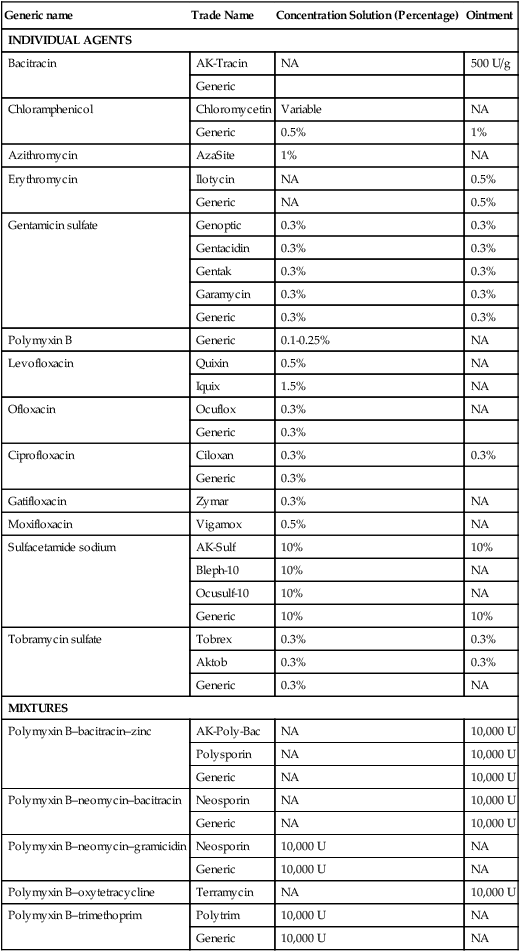
Generic name
Trade Name
Concentration Solution (Percentage)
Ointment
INDIVIDUAL AGENTS
Bacitracin
AK-Tracin
NA
500 U/g
Generic
Chloramphenicol
Chloromycetin
Variable
NA
Generic
0.5%
1%
Azithromycin
AzaSite
1%
NA
Erythromycin
Ilotycin
NA
0.5%
Generic
NA
0.5%
Gentamicin sulfate
Genoptic
0.3%
0.3%
Gentacidin
0.3%
0.3%
Gentak
0.3%
0.3%
Garamycin
0.3%
0.3%
Generic
0.3%
0.3%
Polymyxin B
Generic
0.1-0.25%
NA
Levofloxacin
Quixin
0.5%
NA
Iquix
1.5%
NA
Ofloxacin
Ocuflox
0.3%
NA
Generic
0.3%
Ciprofloxacin
Ciloxan
0.3%
0.3%
Generic
0.3%
Gatifloxacin
Zymar
0.3%
NA
Moxifloxacin
Vigamox
0.5%
NA
Sulfacetamide sodium
AK-Sulf
10%
10%
Bleph-10
10%
NA
Ocusulf-10
10%
NA
Generic
10%
10%
Tobramycin sulfate
Tobrex
0.3%
0.3%
Aktob
0.3%
0.3%
Generic
0.3%
NA
MIXTURES
Polymyxin B–bacitracin–zinc
AK-Poly-Bac
NA
10,000 U
Polysporin
NA
10,000 U
Generic
NA
10,000 U
Polymyxin B–neomycin–bacitracin
Neosporin
NA
10,000 U
Generic
NA
10,000 U
Polymyxin B–neomycin–gramicidin
Neosporin
10,000 U
NA
Generic
10,000 U
NA
Polymyxin B–oxytetracycline
Terramycin
NA
10,000 U
Polymyxin B–trimethoprim
Polytrim
10,000 U
NA
Generic
10,000 U
NA
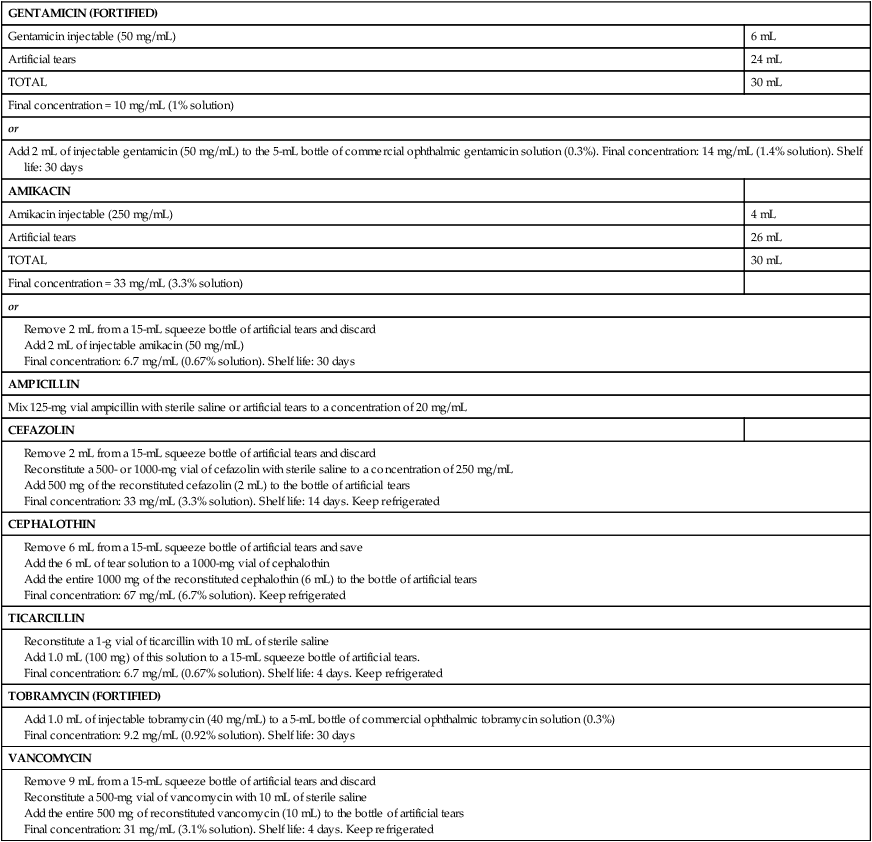
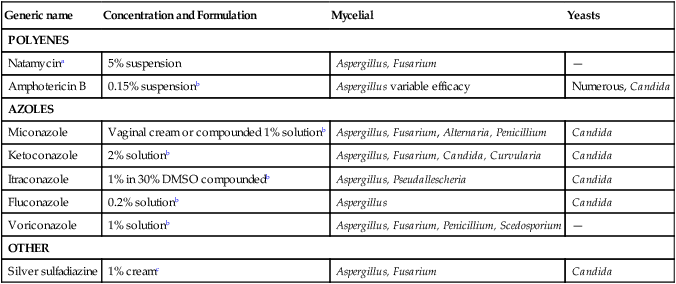
Generic name
Concentration and Formulation
Mycelial
Yeasts
POLYENES
Natamycina
5% suspension
Aspergillus, Fusarium
—
Amphotericin B
0.15% suspensionb
Aspergillus variable efficacy
Numerous, Candida
AZOLES
Miconazole
Vaginal cream or compounded 1% solutionb
Aspergillus, Fusarium, Alternaria, Penicillium
Candida
Ketoconazole
2% solutionb
Aspergillus, Fusarium, Candida, Curvularia
Candida
Itraconazole
1% in 30% DMSO compoundedb
Aspergillus, Pseudallescheria
Candida
Fluconazole
0.2% solutionb
Aspergillus
Candida
Voriconazole
1% solutionb
Aspergillus, Fusarium, Penicillium, Scedosporium
—
OTHER
Silver sulfadiazine
1% creamc
Aspergillus, Fusarium
Candida
Keratitis in Cats
Orbital Infections in Dogs and Cats
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ocular Infections