38 Nutritional considerations in grass sickness, botulism, equine motor neuron disease and equine degenerative myeloencephalopathy
Grass sickness
Grass sickness (GS, equine dysautonomia) is a multisystem neuropathy characterized by damage to autonomic, enteric and somatic neurons. GS has a high mortality rate (>95%) and significant welfare and financial consequences. An identical disorder occurs in dogs, cats, hares, rabbits, llamas (Symonds et al 1995, Whitwell 1997, Lewis et al 2009) and possibly in sheep (Pruden et al 2004).
Epidemiology and risk factors
GS occurs throughout most Northern European countries, with the highest incidence, estimated at 1–2% of horses per annum, being in Northeast Scotland. GS also occurs in Chile, Argentina, Falkland Islands (termed mal seco) and Colombia (termed tambora). Interestingly, despite significant movement of horses between United Kingdom, Ireland, and North America, only one case of GS is reported from North America (Wright et al 2010), and the authors are aware of only a few unpublished cases having occurred in Ireland. This suggests that the occurrence of GS is more dependent on the presence or absence of an environmental factor than on direct transmission of a contagious agent between horses. The temporospatial clustering of GS cases is consistent with involvement of contagious or other spatially and temporally localized processes such as local climate and/or pasture management practices (French et al 2005). Most risk factors for GS (Table 38-1) are consistent with it being a toxico-infection with a resilient soil borne organism, such as Clostridium botulinum, to which horses may develop immunity.
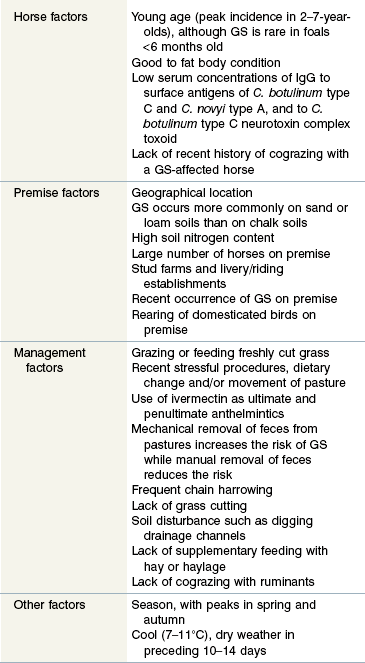
Data from Gilmour & Jolly 1974, Doxey et al 1991a,b, Wood et al 1998, Hunter & Poxton 2001, McCarthy et al 2004, Newton et al 2004.
As the name suggests, GS almost exclusively affects horses that eat fresh herbage, either because they are grazing or because they receive freshly cut herbage when stabled. The risk of GS is greater when horses are grazing full-time than part-time (Gilmour & Jolly 1974), however, even short duration grazing, such as grazing verges while ploughing or while walking in hand, is considered sufficient to induce GS (Begg 1936). All but 2 of 183 GS cases reported by Wood et al (1998) were grazing for at least part of the day, and one of these nongrazing GS cases had been at pasture 1 week previously, while the other case was not confirmed by histological examination of autonomic neurons. There are, however, previous references to GS affecting stabled horses that had no apparent access to fresh herbage (Nairn 1922, Pool 1928, Guthrie 1940), including a group of pit ponies that ate only cereal, bran, and hay (Forsyth 1941). Guthrie (1940) suggested that such cases could result from ingestion of the putative causal toxin in hay or in soil which was adherent to the turnips which were fed to some stabled horses. The latter is consistent with recent proposals that soil, rather than grass, may be the source of the causal agent (Wood et al 1999, Bohnel et al 2003). Unfortunately, these early reports of GS in housed horses are largely anecdotal reports that were not confirmed by histological examination of autonomic neurons. More convincingly, Lannek et al (1961) reported GS in 7 of 36 stabled horses in Sweden which had no access to fresh herbage, with GS being confirmed by histological examination of autonomic ganglia in 1 case. A possible link with ingestion of linseed (Linum usitatissimum) was investigated, but not proven. The occurrence of dysautonomia in housed cats (Symonds et al 1995) and caged rabbits (Whitwell 1997), which likely results from the same neuronal insult as GS (Whitwell 1997), indicate that these diseases can occur in the absence of access to soil, pasture or fresh herbage.
Etiology
While there is increasing evidence that GS is a toxicoinfection with C. botulinum types C or D (see McGorum & Pirie 2009 for review of evidence), definitive proof is currently lacking. Alternative hypotheses include Clostridium perfringens enterotoxicity (Ochoa & Velandia 1978, Gilmour et al 1981) and mycotoxicosis (Robb et al 1997). Cyanide toxicity, resulting from ingestion of cyanogenic glycosides in white clover (Trifolium repens) (Gordon 1934, Greig 1942, Lannek et al 1961, McGorum & Anderson 2002), can be discounted as a cause of GS because the authors have observed GS in horses grazing pastures which have no cyanogenic plants. Toxicity from ingestion of alsike clover (Trifolium hybridum) (Tocher et al 1923) or tormentil (Potentilla tormentilla) (Nairn 1922) has also been discounted as a cause of GS.
C. botulinum serotypes A, B, C, D and E and their neurotoxins have been identified in soil and herbage from GS-affected premises (Bohnel et al 2003). While the presence of botulinum neurotoxins (BoNT) on freshly cut grass appears to contradict the strict anaerobic nature of C. botulinum, it is hypothesized that the bacterium may survive and produce toxins within the protective biofilm coating the leaf surface. The diversity of serotypes identified in the soil was considered as evidence that they were introduced from external sources, such as animal or bird feces or organic fertilizers, rather than representing the geo-specific types of C. botulinum which are normally found in different types of soil (Smith & Sugiyama 1988). If GS is indeed a toxicoinfectious form of botulism, the longevity of botulinum spores in soil (Mitscherlich & Marth 1984) may explain the frequent recurrence of GS on some premises.
Plants collected from fields immediately after an outbreak of GS had reduced antioxidant and weak pro-oxidant activities, increased concentrations of fructose and low-molecular-weight phenolic compounds, significantly more of one amino acid zone (probably valine), significantly less tartaric acid, and a non-significant decrease in ascorbic acid content, when compared with control plants (McGorum et al 2000). This suggests that plants from GS premises may be under oxidative stress, possibly due to chilling, drought or fungal colonization. It is theoretically possible that the altered biochemical content of ingested plants may contribute, directly or indirectly, to the development of GS. Horses with acute GS have alterations in blood/plasma concentrations of several antioxidants, consistent with oxidative stress, the acute phase response and/or the secondary metabolic complications of GS (McGorum et al 2003). While this study found no evidence of systemic macromolecular oxidative damage, macromolecular oxidative damage occurring at the neuronal level could theoretically contribute to GS. Horses with acute GS also have hyperglycemia (Stewart et al 1940) and hyperlipidemia (Milne et al 1990), presumably reflecting negative energy balance. They also have perturbations in plasma amino acid profile resembling those of severe protein malnutrition (McGorum & Kirk 2001). In addition, horses with GS and cograzing healthy horses had depletion of the plasma sulfur amino acids cyst(e)ine and methionine which may indicate xenobiotic exposure.
Clinical signs and diagnosis
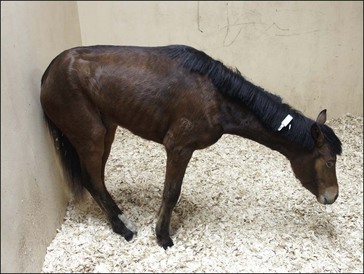
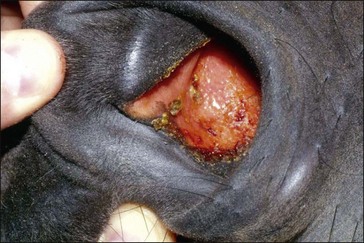
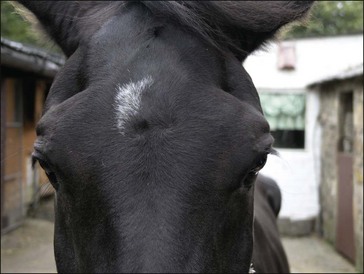
Clinically, GS occurs in acute, sub-acute and chronic forms, reflecting the severity and duration of clinical signs, most of which are attributable to autonomic dysfunction. These include dysphagia, generalized ileus, patchy sweating, salivation, ptosis, rhinitis sicca and tachycardia. The clinical signs and diagnosis of GS are described in detail elsewhere (Pirie 2006, McGorum & Pirie 2009) and are illustrated in Figs 38.1–38.3).
Prevention of GS
Until the cause of GS is known, it is difficult to give sound advice regarding disease prevention. While attempts to eliminate the risk factors listed in Table 38-1 should theoretically reduce the statistical probability of a horse developing GS, prevention of GS on high risk premises is probably achieved only by permanently stabling horses and feeding a diet free from freshly cut herbage. This control measure, however, is rarely used because of practical, economic and welfare considerations. Furthermore, occasional cases have been reported in stabled horses (vide supra). Following the discovery of an association with weather (Doxey et al 1991a), some owners of high-risk premises stable their horses when dry weather with a temperature of 7–11°C has persisted for 10 consecutive days. Stabling is particularly recommended for new horses that are moved onto high-risk premises. If certain fields are considered high-risk for GS, they can be grazed by sheep or cattle, especially in spring. If GS occurs in a group of horses, it is generally recommended that the co-grazing horses are stabled for 2–4 weeks, or moved to another field, because they are otherwise at increased risk of developing GS.
Management of GS
Horses with acute or subacute GS should be euthanized on humane grounds once a confident diagnosis is made, because these forms are invariably fatal. Some horses with chronic GS survive with intensive nursing care (Doxey et al 1995a). Owners should be aware, however, that the survival is largely determined by the severity of nerve damage and the associated complications, rather than the quality of the veterinary and nursing care. Consequently, even with the best care, an affected horse may not survive. As there is no specific antidote or drug treatment to reverse nerve damage, nursing care can only address the common complications of gastrointestinal dysfunction, anorexia, weight loss, dysphagia, dehydration and rhinitis sicca. Analgesics, omeprazole, intestinal prokinetics, fluids, electrolytes and probiotics are commonly administered, but their efficacy is unknown. The reader is directed to other texts for detailed information on management of chronic GS (Milne 1996, McGorum et al 2009). Cisapride, an intestinal prokinetic drug which reduces gastrointestinal transit time in chronic GS (Milne et al 1996), is currently rarely used because of its limited availability, safety concerns and high cost and because its effect on survival rate is unknown. Unfortunately, administration of the antioxidant acetylcysteine, the appetite stimulant brotizolam or a laxative/antioxidant extract from Aloe vera, did not give a measurable increase in case survival (Fintl & McGorum 2002).
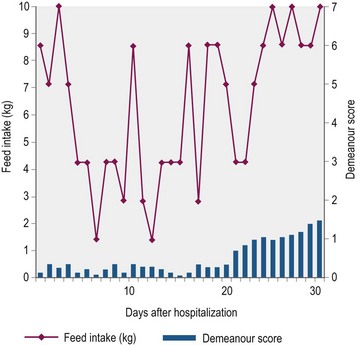
Nutritional support is essential to counteract the marked cachexia which characterizes GS. Unfortunately depression, poor appetite, dysphagia, colic and pain associated with esophageal and gastric ulceration limit the quantity and nature of food that is ingested. Indeed, few horses eat well for more than four days consecutively (Doxey et al 1995b; Fig. 38.4). Ideally, a high-energy, high-protein diet, which is palatable and readily swallowed, should be fed in small amounts frequently throughout the day (4–5 feeds per day). In practice, however, the selection of food offered is usually dictated by the individual horse’s preferences, which unfortunately change regularly, rather than being based on nutritional requirements. Feeds are often offered warm or tepid in the early stages to improve palatability. It is worth offering feeds of different consistency to determine which is preferred, although most horses prefer a mix of soup consistency made by thoroughly soaking pellets in water or dilute molasses. As affected horses rarely eat sufficient conserved forage, particularly if they are dysphagic, they should be allowed frequent short duration access to grazing or offered freshly cut grass. Hay or haylage should be avoided if the horse has a tendency to develop esophageal choke. In the early stages, particularly when horses are depressed, hand feeding from a raised bucket may encourage eating. Hooking the feed bucket on a stable door may also encourage horses to eat and take an interest in their environment.
Most horses that have a realistic chance of survival do not require prolonged administration of fluids and/or feeds via intravenous or nasogastric routes. Short-term nasogastric feeding of pellet slurries has proved beneficial in some cases, but this procedure is poorly tolerated by some horses with severe rhinitis sicca. Extreme care should be taken when administering food by stomach tube to horses with GS because administration of large quantities of high energy, high protein food can induce frequently fatal intestinal bacterial overgrowth, diarrhea and toxemia. Consistent with this observation, small intestinal bacterial overgrowth, commonly with C. perfringens, is one of the major gastrointestinal manifestations of human dysautonomia (Chelimsky & Chelimsky 2003). This is a consequence of gastrointestinal dysmotility and loss of peristalsis which is an important defense against small intestine bacterial colonization (Husebye 2005, Connor & di Lorenzo 2006). The authors have used continuous enteral feeding with Fresubin HP Energy liquid tube feed in four horses with chronic GS. This liquid feed, formulated for treatment of catabolic human patients, was administered via an indwelling narrow bore nasogastric tube (Mila 18 FG), using an Applix Smart pump (Fig. 38.5). As the tube was inserted via a stab incision through the skin overlying the nostril, the horse was able to graze and eat with the feeding system in situ. All three horses had improved demeanour, voluntary food intake and fecal output, without evidence of clinically significant small intestinal bacterial overgrowth or metabolic and electrolyte disturbances resulting from the re-feeding syndrome. Further evaluation in additional cases is required to determine whether this treatment affects case outcome. Inability to maintain the nasogastric tube in situ in some horses appears to be the main limitation of this technique. Close monitoring was required to prevent the tube blocking and the pump dislodging. Parenteral nutrition (see Chapter 41), sometimes in combination with insulin administration, has been used successfully to manage horses with chronic GS for between a few days and several weeks (Andrew Durham, personal communication 2010).
Key Points –
Grass Sickness (GS)
• GS is a multisystem neuropathy, presenting predominantly as gastrointestinal and autonomic dysfunction
• While the etiology is unproven, increasing evidence suggests it may be a toxic-infectious form of botulism type C or D
• Acute and subacute GS is invariably fatal, while horses with chronic GS may recover with intensive nursing care and nutritional support
Botulism
Epidemiology and risk factors
Equine botulism has been associated with four (A, B, C and D) of the seven (A–G) serotypes of C. botulinum, all of which cause an identical clinical disease. Although botulism occurs worldwide, there are geographical differences in the prevalence of equine botulism and in the serotypes which cause disease in horses. In North America, equine botulism is most commonly attributed to serotype B, and less commonly to types A and C (Kinde et al 1991, Wichtel & Whitlock 1991, Whitlock & Buckley 1997, Schoenbaum et al 2000, Weese 2009). C. botulinum grows optimally in anaerobic environments with neutral or alkaline pH, and is inhibited at pH <4.5. BoNT is produced during stationary and growth phases and during bacterial lysis, and optimally at pH 5.7 to 6.2 and when protein concentration is high (Stringer et al 1999). BoNT is inactive until cleaved by bacterial or host proteases.
The three modes of intoxication are ingestion of preformed toxin in contaminated food (forage poisoning), production of toxin within the gastrointestinal tract following ingestion of bacteria or spores (toxicoinfectious botulism or shaker foal syndrome) and production of BoNT from bacteria contaminating wounds (wound botulism). Catastrophic and costly outbreaks of botulism can occur following ingestion of contaminated feeds (Kinde et al 1991). Botulism in adult horses is most commonly due to ingestion of preformed BoNT/B in poorly conserved silage, haylage, hay and straw (Mitchell et al 1939, Ricketts et al 1984, Divers et al 1986, Haagsma et al 1990, Kinde et al 1991). Forage is often contaminated with soil containing C. botulinum spores during harvesting, but clostridia germinate and grow only if the pH is suitable and there is high water content. In good quality silage, microbial fermentation of water soluble carbohydrates produces organic acids, mainly lactic, which lowers the pH to a level which inhibits growth of clostridia. The pH does not drop sufficiently if the forage has low soluble carbohydrate content. While pH ≤4.5 usually inhibits clostridial growth, inhibition may not occur at this pH if the forage has excessively high water content. For this reason, and because clostridia grow in very wet conditions, wet preserved forages pose a risk for botulism. Wet forage should be prewilted under good weather conditions to ensure sufficient acid production to inhibit C. botulinum. It is generally recommended that bagged forage is consumed within 3–7 days of opening the bag because, once the bag is opened, aerobic microbial putrefaction degrades organic acids, increasing the pH and permitting growth of C. botulinum
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree