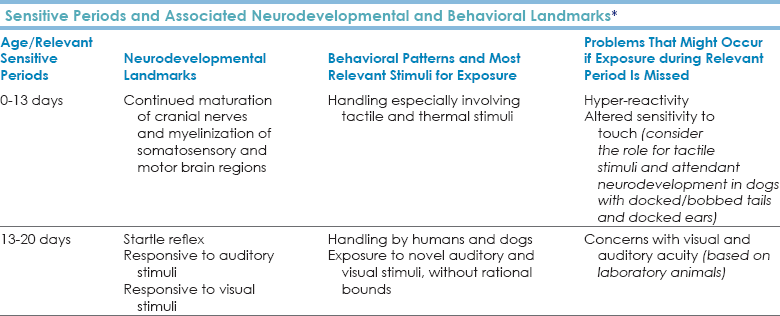
Chapter 4 Molecular data support that dogs separated from wolves 15,000 to 135,000 years ago (Cadieu et al., 2009; Leonard et al., 2002; Lindblad-Toh et al., 2008; Pang et al., 2009; Parker et al., 2004; Savolainen et al., 2002; Sutter et al., 2004; Vila et al., 1997; Vonholdt et al., 2010). Molecular and anthropological data support that dogs of different morphologies who were likely engaged in different tasks have lived together with humans for at least 15,000 years (Boyko et al., 2009; Castroviejo-Fisher et al., 2011; Morey, 1994; Pang et al., 2009). Stand-alone anthropological evidence supports that dogs have lived intimately with humans for at least 30,000 years (Bienvenido et al., 2009; Derr, 2012; Germonpré et al., 2009, 2012; Ovodov et al., 2011). For the past 3500 years or more (consider dogs portrayed in ancient Chinese and Egyptian art), there have been well-defined breed clusters or groups comprising dogs of different shapes and sizes who engaged in related tasks. Regardless of the debate over timing, we should appreciate that one of the forces associated with speciation may have been a special, collaborative working relationship with humans that ultimately resulted in morphological variation in dogs as a relatively—perhaps profoundly—late development in the human × dog relationship. We accept that humans have changed dogs. We seldom consider the extent to which dogs may have changed humans. Our unique relationship with dogs may be due to convergent evolution of canid and human social systems that was the result of like groups meeting and recognizing the power of collaborative efforts, followed by secondarily derived, homologous changes in brain function (Saetre et al., 2004) that have allowed modern humans and dogs to rely on each other. Dogs, as a species separate from wolves, likely co-evolved with humans over thousands or tens of thousands of years, during which time they may not have been fully “domesticated.” “Domestication” may have occurred when we began to develop breed groups intended for specific tasks, which happened 3000 or more years ago. In contrast to wolves, who require handling by humans early in their ontogeny (beginning by at least 14 days of age) to minimize fear and reactivity to humans, most dogs can adapt to delays in handling and exposure, and their normal, innate sensitive periods are greatly expanded from those of wolves. When dogs cannot adapt or when they seem to have short sensitive periods, such patterns are indicative of pathology. In dogs, it is likely that this sensitive period expansion occurred concomitantly with changes in molecular and neurochemical function and gene expression (Saetre et al., 2004) that may represent some of the true outcomes of active “domestication.” In contrast to dogs, but similar to other non-domesticated species, cats have a relatively short and truncated sensitive period for exposure to humans, which may be associated with or evidence of lack of tampering associated with “domestication.” Both humans and canids live in extended family groups, provide extensive parental care, share care of young with both related and non-related group members, give birth to altricial (completely dependent, immature) young that require large amounts of early care and sustained amounts of later social interaction, nurse for an extended period before weaning to semi-solid food (dogs do this by regurgitation; humans use baby food, but the concept is the same), have extensive vocal and non-vocal communication (it has been estimated that 80% of all human communication is nonverbal) (Smith, 1965, 1977), and have a sexual maturity that precedes social maturity. These shared characteristics may have allowed dogs and humans to recognize similarity in each other that allowed exchange of information and that led to later “domestication” and changes on the parts of both dogs and humans related to task management. Early Brain and Behavioral Development in Dogs Myelination of cranial nerves V (trigeminal), VII (facial), and VIII (vestibulocochlear/auditory) is present at birth. These nerves are associated with essential functions of eating, balance, and body-righting. As is true for other animals, myelin is almost completely absent from the brains of newborn puppies but appears during the first 4 weeks, at the same time ribonucleic acid (RNA) synthesis is increasing at a rapid rate (Fox, 1971). In humans, myelin is first deposited in the peripheral nervous system (PNS), the central nervous system (CNS) in the brainstem and cerebellum, and in components of some major motor systems just before and after birth. Myelination of the human brain cortices occurs well after birth and progresses over decades (Volpe, 2008). Dogs undergo a gradual increase in myelination of the spinal cord, motor and sensory roots, and efferent pathways starting at birth and progressing through 3 weeks, which is reflected in the development of olfactory, thermal, and tactile capability and in increased mobility associated with development of vision. PNS and CNS development is reflected in development of motor responses and reflexes in young pups. This period of gradual myelination is followed by more rapid myelination of the somatosensory cortex at about 4 weeks and a more even distribution of myelination of the visual and auditory cortex by 6 weeks (Fox, 1971). As with humans, myelination is slowest in the frontal lobe. As brain development progresses, canine behavior becomes more complex, and the markers for onset of “socialization” or sensitive periods appear to be neurodevelopmental. Much of what is known about early social development is the result of work done by Scott et al. in a laboratory on five breeds of similarly shaped dogs (wire-haired terriers, Shetland sheepdogs, cocker spaniels, beagles, and basenjis), using a relatively small number of litters over 2 decades and observing pups beginning at 3 weeks of age (Scott and Fuller, 1965). These studies remain landmarks. We still lack comparable data on most breeds, yet such data could be relatively easily collected across breeds, as demonstrated by Schoon and Goth Berntsen (2011), who provide excellent data on neurodevelopmental landmarks from birth for 10 litters of Belgian malinois raised under controlled conditions. The following broad conclusions appear to hold for most dogs: • The “neonatal period” covers the period from birth to 13 days of age when puppies are dependent on rudimentary locomotor skills and use tactile signals to locate and orient toward dams and littermates. During this period, puppies vocalize if separated from their dams. Olfactory ability is present but poorly characterized in dogs this age. Few data about tactile signaling exist, yet given its early importance it should help structure brain development. • The mild stress of daily and early handling is beneficial for puppies and allows them to cope better with later stresses (Selye, 1952). Excessive stress should be avoided because chronic, excess secretion of adrenocorticotropic hormone (ACTH) has been correlated with a decreased ability to learn. • From days 13 to 20, puppies become more coordinated, open their eyes, and begin to startle to sound. The change in motor abilities coincides with eruption of teeth at approximately day 20 and with improved vision. This period is traditionally called the “transition period.” • Tail-wagging behavior becomes apparent at the end of this 20-day period, and there is considerable variation across breeds in this development. No one has investigated the extent to which use of the tail in signaling may reflect an effect of neurodevelopment but this is an important question. • If pups are exposed to passive observers beginning at 3 weeks of age, they will approach and explore the observer. • If pups are not exposed to passive observers until 7 weeks of age, they must habituate to the observers before they approach and explore. This habituation took 2 days in the laboratory setting (Bacon and Stanley, 1963, 1970; Freedman et al., 1961; Love and Eisenberg, 1986; Scott and Fuller, 1965). • Dogs isolated from humans through 20 weeks became fearful of humans (Agrawal et al., 1967) and had impaired learning ability (Melzack, 1968; Melzack and Scott, 1957; Thompson and Heron, 1954). • Even if kept with their mothers, by 12 weeks of age, puppies chose to wander extensively, a finding that anyone who has raised puppies has witnessed. • Pups that were kept in kennels beyond 14 weeks were very timid and demonstrated a lack of confidence in any circumstances other than the kennel. These dogs would not voluntarily leave the kennel and became truly phobic of anything novel (neophobia) (see Scott and Fuller, 1965, for summary data). • Different breeds responded differently to various rewards and restraints and differently with respect to various social contexts (Plutchik, 1971), and these patterns were replicable. Based on these data, the period from 3 to 12 weeks was called the “socialization period,” from which a number of context-specific developmental periods were identified as “socialization periods.” These periods became prescriptive with respect to types and extent of exposure that were thought to be required to produce “normal dogs.” Instead, such data are best viewed within the context of a “sensitive period” (Bateson, 1979), which implies risk assessment. There are environmental and genetic aspects of all behaviors (see Figures 3-3 and 3-4 accompanying discussions in Chapter 3), and some individuals may benefit from earlier exposure than others. If the opportunities are available, non-pathological dogs will expose themselves when they are able. A concept of a sensitive period takes such variation into account and is best defined as period when animals can best benefit from exposure to certain stimuli, and if deprived of such exposure, there is an increased risk of developing problems attendant with the stimulus. In other words, when animals are neurodevelopmentally able to respond to stimuli, they will benefit from exposure, and if they lack exposure, they could develop behavioral problems associated with the omission (Bateson, 1979; Cairns et al., 1985). This does not mean that all exposure is equal, that all dogs are ready for all exposures at the same time, that you stop exposing the dog when the dog is out of the sensitive period, or that if exposed, no dogs will have problems. We also should remember the role for cortical development in how a puppy learns to respond to different stimuli and understand that the time/developmental period of imposed change matters to the dog. A study comparing 70 adult dogs who as puppies had been separated from their dams and litters 30 to 40 days with 70 adult dogs who as puppies were not separated until after 8 weeks showed that early age of separation was a significant predictor for excessive barking, fearfulness on walks, reactivity to noises, toy possessiveness, food passiveness, and attention-seeking behavior. These dogs were also more at risk for destructive behavior than dogs who had been permitted to stay with their litter through 8 weeks (Pierantoni et al., 2011). Clearly, there are potential roles for both the hormonal effects of stress/distress and the developmental phase in these findings. Considering the enhanced risk of relinquishment, abandonment, and euthanasia for dogs with behavioral concerns, welfare and behavioral standards should mandate that puppies remain with their litters in the home of and with access to the dam through 8 weeks of age (Box 4-1). General guidelines for exposure based on the available data are found in Table 4-1. TABLE 4-1 Sensitive Periods and Associated Neurodevelopmental and Behavioral Landmarks* *These periods indicate when dogs are first most receptive to the noted stimuli. There is no implication that dogs ever stop learning from their experience. Data support the concept of repeated, ongoing neurological and molecular remodeling with exposure, as occurs in humans. Play appears to be important in every species in which it has been studied. Although play has been thought to have numerous roles in behavioral development and maintenance from enhancing coordination and locomotor activity to encouraging problem-solving ability and enhancing cognition (Spinka et al., 2001), it may be especially effective in teaching animals how to make mistakes successfully and in established baselines for well-honed, broad, basic communication skills. This hypothesis is supported by data showing that dogs who received more playful interactions from their people were less fearful in new environments (Tóth et al., 2008). In dyadic relationships of dogs participating in free play, sex of participants does not affect play, but age does: older dogs play more forcefully than younger dogs (Bauer and Smuts, 2007). Play and play signals also appear to modulate interactions between younger dogs and more forceful older dogs in dyadic play. Play signals given by younger dogs alter the course of more forceful interactions by older dogs (Bauer and Smuts, 2007), likely by making intentions more clear. Dogs playing with other dogs play with toys differently than dogs playing with humans. When dogs play with humans, they are more interactive and less likely to continue to hold the toy (Rooney et al., 2001). Humans play with dogs using vocal, tactile, and visual/postural cues that can affect how dogs play. When humans display the lunge and bow aspects of the canine play bow, dogs increase play, and lunging increases play duration and frequency (Rooney et al., 2001). If the humans added vocal signals, play was enhanced. Effective human play with dogs enhances the relationship between the dog and the human, reduces the incidence of behavioral problems, and encourages humans to think that their dogs are very clever (Rooney and Bradshaw, 2002, 2003). Play signals affect how dogs interpret the information provided by interactions between humans. In staged contests between humans, dogs whose humans give play signals approach more quickly than dogs whose humans provide no signaling information (Rooney and Bradshaw, 2002). Because play behavior involves a number of signaling modalities (vocal, visual, tactile, olfactory [licking, sniffing]), the redundant signals involved minimize the risk of mistakes in communication and help young animals learn about managing mistakes and quick changes in interactive behavior. Dogs should be allowed sufficient safe access so that when they enter their individual sensitive periods, there is no impediment to them exploring the relevant environment or having the relevant social interactions. The earlier dogs can learn about the broad-scale social and physical environment in which they are to live, without inducing fear, the better. If dogs are protected from stimuli, they may react inappropriately when exposed later (Scott, 1963; Bacon and Stanley, 1970). If the pups had healthy dams, are healthy themselves, and are engaged in a modern vaccination program, they can be exposed to as many situations as possible. Of 24,000 guide dog puppies who began vaccination at 6 weeks of age and were re-vaccinated every third week through weeks 12 to 16, fewer than 6 pups ( Very early fear is a problem for pups. Historically, pups from lines of dogs genetically selected to show fearful behaviors show the behavioral and physiological effects of fear by 5 weeks (Murphree et al., 1967, 1969). Pups from lines of dogs commercially bred for research purposes show almost the same distribution of fearful behaviors at 5 weeks of age as do their dams and sires at 1.5 to 2 years (Overall et al., unpublished). Puppies who are shy, worried, or anxious throughout early veterinary visits are likely to exhibit the same behaviors as adults at 1.5 to 2 years of age (Godbout et al., 2007). Early intervention is essential for such dogs. One study has suggested that there could be a beneficial effect of pheromonal analogue collars on one of a series of behaviors studied, early excitability in class, and hence on learning in puppy classes, based on owner surveys (Denenberg and Landsberg, 2008). Even if this effect were real—and the data, techniques, and analysis are problematic (Frank et al., 2010)—the magnitude of the effect appears mild, especially considering that it uses a tool (ranks of owner responses) that may exaggerate mild and/or rare outcomes. There are no data to suggest that pheromonal products are helpful for early fears, and so treatment with interventions whose mechanisms are known to work should not be delayed. Early intervention, often involving medication, is essential for these dogs so that they have a decent quality of life. Claims have been made that some types of handling and stressors, including those discussed in early neural stimulation, protect dogs from effects of later stress (Battaglia, 2009). Only one study has evaluated early neural stimulation in a blinded, controlled, rigorous manner, and this study showed that it had no effect on the dogs chosen for the evaluation (Schoon and Goth Berntsen, 2011). However, as the authors note, the dogs tested were purpose-bred to become mine-detection dogs and already lived in an extremely enriched environment where they were intensely handled and interacted with daily as part of their routine protocol. This is exactly the environment in which you would expect not to see an effect because the control dogs are also highly, albeit slightly differently, stimulated. For dogs raised in homes, kennels, or commercial breeding facilities where dogs are behaviorally deprived, early stimulation of any kind is known to be beneficial. The time between the end of myelination of the cortex and concomitant development of normal social and exploratory behavior (8 to 12 weeks) and the development of sexual maturity is considered to be the “juvenile” period (Scott and Fuller, 1965). Dogs are sexually mature by 6 to 9 months of age. If the dog is to be a breeding dog and does not show signs of sexual maturity by 6 to 9 months of age, further consultation is warranted. Sex hormones may interact with various neurodevelopmental systems, but no data exist on the effects of early versus later neutering on these systems in dogs, with the possible exception of correlates on long bone growth. Regardless, both neutered and intact animals are affected by behavioral concerns and pathologies. We lack information on dogs that is now available for humans (and rodents), but it may be safe to assume that myelination and neuronal pruning occur rapidly for the first few months of life and then slow until social maturity, as is the pattern in humans. Social maturity is a period of renewed but progressive myelination and regressive pruning that is associated with changes in neurochemical profiles and shifts in behavior (Sowell et al., 1999). Behavioral changes attendant with canine social maturity begin at approximately 12 to 18 months. There is likely considerable variation attached to this estimate because of breed and size differences and because some dogs are still bred and selected for certain tasks. Such concerns are reduced for humans. Humans are sexually mature at some point between 8 and 13 years of age, but are not socially mature—based on functional imaging and neurochemical evidence—until well into their 20s or 30s. As humans age from 6 to 17 years, cerebral gray matter volume decreases, and white matter and corpus callosum volumes increase, suggesting increased ability to integrate and act on information, when measured by magnetic resonance imaging (MRI) (De Bellis et al., 2001). Standard MRI assays reveal profound differences in size-by-age trajectories of brain development between males and females as they mature through age 27 years (De Bellis et al., 2001; Lenroot et al., 2007). Using diffusion tensor MRI, age-related (5 to 30 years) changes are seen in many areas of the human brain (i.e., deep gray matter, subcortical white matter, major white matter tracts) in a pattern that suggests that connections between the frontal and temporal lobes develop more slowly than other regions. Patterns of change in maturation of the human frontal cortex appear to improve cognitive processing, a hypothesis supported by congruent data from electrophysiological, positron emission tomography, and neuropsychological studies on normal cognitive and neurological development (Sowell et al., 1999). Trajectories for regional brain maturation can be affected by trauma, indicating how important connecting tracts are in the development of adaptive behaviors. Children with post-traumatic stress disorder (PTSD) have larger prefrontal lobe cerebrospinal fluid volumes and smaller regional measurements of the corpus callosum than age-matched unaffected children; this finding is amplified for boys with PTSD (De Bellis and Kreshavan, 2003). We should hypothesize that the same pattern can occur with dogs. Deferential relationships in neither dogs nor humans are structured as linear hierarchies. Most concepts involving “dominance” in dogs are outdated, something that the behavioral community has finally recognized (American Veterinary Society of Animal Behavior [AVSAB] Dominance Position Statement: www.avsabonline.org/avsabonline/images/stories/Position_Statements/dominance%20statement.pdf and the Dog Welfare Campaign position statement: www.dogwelfarecampaign.org/why-not-dominance.php). Many situations in which “dominance” is implicated in hierarchies may be artifacts. The study of relationships between fewer than six animals will automatically produce a numerical rank order hierarchy that is linear (Bernstein, 1981; Boyd and Silk, 1983; Rowell, 1974; Syme, 1974), but the ranks produced are unable to account for the social complexities that are noted. Instead, deferential behaviors are dependent on context and are based on knowledge, age, size, and the situation in which individuals are interacting. More information in language useful for clients can be found in the client handout, “Protocol for Generalized Discharge Instructions for Dogs with Behavioral Concerns.” It is not surprising that humans were able to incorporate dogs into our social groups, as we were incorporated into theirs. Understanding Non-vocal Signals Because dogs and people do have such similar social systems and use so many of the same signals, it has been very easy for people to assume that when a dog gives a signal that resembles a human signal, the message is exactly the same and that it means exactly the same thing (Smith, 1965). We have shared signals, but we should also understand that animals who do not use verbal speech in the manner we do and who do not have opposable thumbs may have their own signals that we could benefit from learning, rather than always expecting us to learn their signals. When humans allowed dogs to teach them to play using canine signals (bow and lunge), the relationship between the human and the dog improved, and dogs who had not played now played enthusiastically (Rooney and Bradshaw, 2001). Behavioral descriptions are usually made on the basis of either structure (e.g., descriptions of postures or sounds) or consequences (e.g., the effect of the behavior on the individual exhibiting the behavior, others in the behavioral environment, and the behavioral environment itself). A good description of these concepts can be found at the Animal Behavior Society website (www.animalbehavior.org/ABSEducation/laboratory-exercises-in-animal-behavior/laboratory-exercises-in-animal-behavior-ethograms). The difficulty in veterinary behavioral medicine often arises when descriptions of measures and consequences are confused or given an associational or causal link without actually testing whether that link is valid or true. For descriptors to have scientifically valid causal or associational links, we would have to measure or assess whether these terms characterize what they were intended to characterize, an effort that is seldom made. Early canine behaviors can be divided into et-epimeletic (care-seeking), epimeletic (caregiving), and allelomimetic (group-activity) behaviors. Until about 4 weeks of age, the relationship between the mother and puppies is primarily epimeletic. Box 4-2 contains the traditional classification of these stage-associated behaviors. Many of these behaviors are also seen later in life and can be understood by referring to this ontogenic history. Canine Visual Communication—What Can Dogs See? Eye radius, a measure of size, positively correlates with the length and width dimensions of the skull but not with cephalic index, a measure of shape, rather than size (skull width/skull length × 100). Among breeds of domestic dogs, retinal ganglion cells range in distribution from a dense concentration in a strong area centralis, with virtually no visual streak, to a strong, horizontally distributed visual streak and almost no area centralis (McGreevy et al., 2004). • Skull length was negatively correlated with peak area centralis density of ganglion cells but positively correlated with peak density of ganglion cells in the streak (e.g., long-nosed dogs have few ganglion cells in the area centralis but a lot of ganglion cells in the streak). • The ratio of ganglion cell densities in the area centralis to cells in the streak was negatively correlated with skull length (i.e., the more cells in the area centralis compared with the streak, the shorter the skull). • Dolichocephalic dogs have strong visual streaks and relatively low densities of ganglion cells in the area centralis, and brachycephalic dogs have concentrated ganglion cells in the area centralis and low to no concentrations in a visual streak. • Red/green cones (medium to long wave) were denser in the temporal region than in the area centralis in brachycephalic dogs and less concentrated in the temporal retina in dolichocephalic dogs. Blue cones (short wave) were sparse compared with red/green cones in all dogs. • The number of ganglion cells correlates positively with skull size. McGreevy et al. (2004) did not examine the extent to which skull shape and size may have affected distribution of olfactory neurons, but it may be prudent to expect an effect. Dogs see in rudimentary color vision (dichromatic), and they are sensitive to short-wave (bluish) light. Dog color vision is sufficiently discriminating so that they can pick out an object based on color (Neitz et al., 1989). Dogs have two classes of photopigment in cones with spectral peaks at approximately 429 nm (blue) and 555 nm (green), the peak of sensitivity for light-adapted eyes. In bright light, however, dogs have less acute vision than humans. Rhodopsin in dogs has a peak sensitivity to wavelengths of 506 to 510 nm and requires more than an hour to regenerate completely after exposure to bright light. The extent to which rods and cones develop is dependent on early exposure to a full range of ambient light conditions and to critical nutrients docosahexaenoic acid (DHA).
Normal Canine Behavior and Ontogeny
Neurological and Social Development, Signaling, and Normal Canine Behaviors
Overview of Normal Dog Behavior
Behavioral Ontogeny in Dogs
Roles for Play
Early Exposure, Puppy Classes, and Vaccination Programs
 of 1%) who were healthy during the vaccination series got sick (Appleby, 1993). If there are available puppy classes or puppy play groups, any pup physically and behaviorally able to participate should do so. If pups shy from these groups or classes and gentle continued exposure does not alleviate their response, they need help immediately.
of 1%) who were healthy during the vaccination series got sick (Appleby, 1993). If there are available puppy classes or puppy play groups, any pup physically and behaviorally able to participate should do so. If pups shy from these groups or classes and gentle continued exposure does not alleviate their response, they need help immediately.
Brain Changes and Social Maturity
Why It Is Not about “Dominance”
How Dogs Communicate
Early Behaviors
Systematic Approach to Understanding Canine Signals
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine
