and Paul B. S. Clarke1
(1)
Department of Pharmacology & Therapeutics, McGill University, Montreal, QC, Canada
Abstract
Intravenous self-administration (IVSA) studies have shown that nicotine can serve as a reinforcer in animals and humans. Brain mechanisms underlying nicotine IVSA, as well as the effects of pharmacological interventions, have also been widely investigated and are summarized. However, the conditions under which nicotine self-administration has been observed do not closely model the way in which nicotine is consumed by cigarette smokers, in two important respects: conventional IVSA procedures typically employ ultra-rapid infusions and high unit doses of nicotine that approximate the content of one or two cigarettes. In contrast, recent evidence shows that rats will also self-administer nicotine in doses equivalent to about two cigarette puffs, delivered into the circulation at a much slower rate that more closely approximates the delivery rate in cigarette smokers. Possibly, adoption of these refinements will help to provide an animal model with greater predictive clinical validity in order to advance the search for more effective smoking cessation pharmacotherapies.
Key words
NicotineTobaccoIntravenous self-administrationSelf-administrationReinforcementReviewNicotinic receptorsAnimal models1 Scope
It is widely believed that cigarette smoking stems largely from nicotine addiction. Reinforcing effects of nicotine have been extensively investigated using the nicotine intravenous self-administration (IVSA) procedure in adult rats. After describing methodological aspects of the technique, we highlight its main applications in this species, particularly related to pharmacotherapy development. Finally, we discuss further refinements of the procedure that may provide a closer model of human cigarette smoking. (Please note that we will use the terms “dependence” and “addiction” interchangeably in this chapter.) Important research has also been performed in other species, notably squirrel monkeys (1, 2), rhesus monkeys (3, 4), and in wild type and genetically modified mice (5–7). These species fall outside the scope of this review and the reader is referred to recent reviews by others (8, 9).
2 The Problem of Tobacco Addiction
Tobacco dependence is a major preventable cause of death. In developed countries, tobacco smoking is estimated to cause about 30% of all deaths from cancer, and an even greater number of deaths from other diseases (10). Globally, smoking accounts for 4–5 millions deaths each year (10). The primary problem facing smokers who attempt to quit is the high rate of relapse and the poor effectiveness of current pharmacological and non-pharmacological therapies (11). A better understanding of tobacco dependence appears necessary in order to provide more effective pharmacotherapies.
3 Tobacco Dependence versus Nicotine Addiction
Cigarette smoke contains about 4,000 identified chemicals, the great majority of which have never been characterized pharmacologically or behaviorally. Nevertheless, the 1988 US Surgeon General’s Report concluded that “nicotine is the drug in tobacco that causes addiction” (12). This widely held view has provided the rationale for well over 100 published reports of nicotine self-administration. Indeed, nicotine IVSA is currently the dominant animal model related to cigarette smoking. Recently, nicotine’s central role in tobacco dependence has been vigorously questioned (13, 14), and a more nuanced view appears to be emerging (15, 16).
4 Oral Nicotine Self-Administration in the Rat
Most nicotine self-administration studies employ intravenous delivery, but there are also several published reports of oral self-administration. The latter approach offers several advantages: catheterization surgery is avoided, extensive training is not required, 24-h access is convenient, and long-term testing is straightforward. One obvious drawback is that nicotine absorption is slow and potentially variable. Moreover, since nicotine solutions are bitter, rats need to be encouraged to consume nicotine solutions, either by schedule-induced polydipsia (17), sweetening with sucrose (18), or by gradually increasing the nicotine concentration (19). Results to date are somewhat equivocal. For example, in one study, rats lever-pressed more for oral solutions of nicotine + sucrose than of sucrose alone, yet drank less when consumption was unrestricted. In a further experiment, rats could choose to respond for either aqueous nicotine solutions or water; weaker nicotine solutions were mildly preferred, whereas stronger solutions were avoided (18).
5 What Purpose Do Intravenous Nicotine Self-Administration Studies Serve?
Cigarette smoking is a complex behavior modulated by pharmacological, psychological, social, and economic variables. Which aspects of this behavior, then, does nicotine self-administration model? In general terms, the self-administration procedure has been used to model the acquisition, maintenance, extinction, and reinstatement of nicotine-taking behavior. Self-administration behavior, tested in limited-access sessions, is presumably controlled by the acute positive consequences of drug administration. With more extended daily exposure, withdrawal signs can emerge (20, 21); here, negative reinforcement may play an important role. Importantly, nicotine self-administration behavior tends to be weak unless the drug is paired with sensory cues, and it has been proposed that animals self-administer intravenous nicotine primarily because the drug makes these ancillary cues more reinforcing (see below).
Although compulsion is a hallmark of drug addiction, nicotine self-administration studies to date have provided little if any evidence of compulsive drug seeking (but see (20, 21)). However, most studies of nicotine self-administration are quite possibly not long enough for compulsive behavior to manifest itself. It is worth noting that even with cocaine, compulsive drug-seeking behavior can require several months of repeated exposure and/or testing before motivated drug seeking increases sufficiently to be regarded as compulsive (22, 23).
Self-administration studies have been instrumental in identifying molecular and neuronal mechanisms that are critical to the acute reinforcing effects of nicotine. Nicotinic receptors are widely expressed in both the periphery and CNS, but it is the central action of nicotine that appears important to self-administration (24). Several brain mechanisms have been identified that are critical to nicotine IVSA, as described below. In the search for more effective pharmacotherapies for cigarette smoking, numerous drugs have been tested in animals self-administering intravenous nicotine. However, more testing will be necessary to determine whether the standard nicotine self-administration procedure can usefully predict clinical efficacy (25, 26).
6 Do Human Subjects Self-Administer Nicotine?
The Surgeon General’s 1988 conclusions relied quite heavily on two published reports of intravenous nicotine self-administration in human subjects (27, 28). However, these studies have been strongly criticized on a number of grounds (13). First, few subjects were tested in any condition; several had a history of drug abuse. Second, few subjects appeared to self-administer more nicotine than saline. Third, several reported observations about nicotine self-administration were not sufficiently supported by data. Fourth, subjects were told that they might receive nicotine, but nevertheless identified the drug as cocaine. This last observation might be related to the rapidity of drug delivery (9 s), or the use of unit doses (approx. 10–40 μg/kg) that far exceed the nicotine yield from single cigarette puffs (approx. 1–2 μg/kg) (29).
Two recent reports provide stronger evidence of IVSA in human smokers (30, 31). In one of these studies (31) subjects were willing to respond 1,600 times for each nicotine infusion and responded very little for saline infusions. However, several subjects were past or present drug users, and it is not clear how far these results would generalize to the average cigarette smoker (31). A further complication in human IVSA studies is that nicotine produces a recognizable cue, and many subjects believe that nicotine is addictive. Therefore, nicotine self-administration, where observed, could potentially be generated by expectation, unless this possibility is explicitly ruled out (13, 32).
Human subjects have also been tested for self-administration of nicotine in the form of nasal spray and polacrilex gum. In several acute studies, Perkins et al. have compared self-administration of nicotine versus placebo nasal spray in a forced choice task; overall, fewer than half of the smokers chose nicotine consistently over saline, even when abstinent from cigarettes (33, 34). However, a longer-term study suggested that reinforcing effects of nicotine nasal spray may take several days to emerge (35). Similarly, most subjects prefer placebo chewing gum to nicotine gum in acute tests (36, 37), whereas nicotine gum is preferred with prolonged access (35).
In summary, a clear demonstration of nicotine self-administration in humans remains elusive (32), although animals will clearly self-administer the drug. Animal models of drug self-administration offer some significant benefits; for example, more rigorous controls are possible, and the problems of past drug history and biases based on expectations are avoided. The following sections deal with self-administration of nicotine in animals and will be focused primarily on one animal – the adult rat.
7 Intravenous Nicotine Self-Administration in the Rat: Methodology and Early Studies
The IVSA procedure represents the gold standard for studying the motivational aspects of a drug in animals. This procedure possesses significant face validity (38), and it results in patterns of intake that closely match human drug consumption (39–42). In addition, the progressive ratio schedule of reinforcement provides a measure of how motivated the animal is to obtain drug infusions (43). In this procedure, the amount of work required to receive each successive infusion of a drug increases exponentially. At some point, the response requirement becomes too high and the animal ceases to respond for the drug, and thus, the amount of responding required to obtain the last infusion is termed the “breakpoint.” Other drugs can be tested within this procedure to measure any change in the motivation to self-administer a particular drug (see (44) for a review).
The standard nicotine IVSA procedure in the rat is essentially the same as that for other drugs of abuse. Under general anesthesia, the animal is catheterized in the right jugular vein and the catheter is passed subcutaneously to a cannula that is either fixed to the skull or positioned to exit between the scapulae. Catheterization surgery is described in detail elsewhere (45). In order to obtain nicotine infusions, the animal makes an operant response. This response may be a lever press, a nose-poke entry into a receptacle, or movement of a wheel manipulandum. During the self-administration session, a houselight may be illuminated. The infusion is almost invariably coupled with a sensory cue, usually a light placed above the lever or an auditory signal (i.e., a tone). Such sensory cues play a critical role in nicotine self-administration, as discussed below. Following the infusion, a timeout period is typically imposed, lasting from seconds to minutes, during which no further infusion is available; this period serves to minimize aversive drug effects and prevent overdose. Usually, a second, “inactive” lever is provided; responses on this lever do not result in drug delivery and instead serve as a control for general increases in activity caused by the drug or by some other experimental manipulation.
The first demonstrations of intravenous nicotine self-administration in rats employed a variety of methods. For example, Singer et al. obtained high levels of nicotine self-administration by using a procedure normally associated with schedule-induced polydipsia (46). Accordingly, rats were food-deprived and placed on a fixed interval 60 s (FI 60) schedule of food delivery. Cox and coworkers provided an early demonstration of IVSA using a more conventional approach (47). These authors showed that rats would press a lever to obtain intravenous nicotine at doses of 10 and 30 μg/kg (but not 3 μg/kg), and would redirect their responding when nicotine was reassigned to a previously inactive lever. Nicotine self-administration did not require schedule induction, pre-exposure or food restriction. However, only a subset of the rats responded preferentially for nicotine in a consistent manner.
8 Intravenous Nicotine Self-Administration in the Rat: The Corrigall–Coen Procedure
Nicotine self-administration was further refined and characterized by DeNoble and Mele, in a paper whose publication was involuntarily delayed by 20 years (48). Meanwhile, in 1989, Corrigall and Coen described a nicotine IVSA procedure that has served as a template for many subsequent studies (49). Their seminal paper described robust self-administration of nicotine across a range of doses and across various schedules of reinforcement (see Fig. 1).
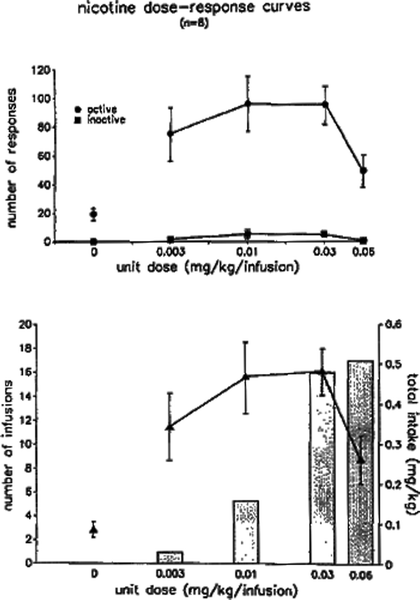

Fig. 1.
Dose-response curves for intravenous nicotine self-administration in limited-access sessions. Adult rats were first trained to lever-press for infusions of 0.03 mg/kg on a fixed-ratio 5 (FR5) schedule of reinforcement, in daily 1-h sessions. Once responding had achieved stability across days, each rat was tested across a range of infusion doses. Each infusion dose was available for several consecutive sessions, and the data shown refer to responding that had stabilized for the dose in question. Both responding (upper panel) and number of infusions (lower panel) were related to unit dose according to an inverted-U function. Total nicotine intake per session increased monotonically with unit dose (lower panel, columns). Data are mean ± SEM (Taken from (49). With kind permission).
The refinements associated with the Corrigall and Coen (1989) procedure are as follows:
1.
2.
3.
Nicotine solutions are pH-neutralized. This is presumably important, because nicotine is commonly used as a bitartrate salt, which forms highly acidic aqueous solutions.
5.
6.
On fixed-ratio schedules, peak rates of responding and number of infusions are typically obtained at doses of 10–30 μg/kg/infusion (20, 51–53, 55, 57, 60, 66, 69–71). However, compared to other drugs such as cocaine and opiates, fixed-ratio responding appears less sensitive to infusion dose (72). In contrast, on a progressive ratio schedule, responding and nicotine intake increase monotonically with infusion dose (73, 74).
Several intrinsic factors affect rates of nicotine IVSA in rodents, including genetic strain (57, 60, 65, 76) and sex (63, 70, 77). Developmental stage also plays a role, and several groups have reported that adolescent rats self-administer intravenous nicotine more than adult rats (58, 76, 78). However, when response demands are increased, adolescent rats may actually self-administer less than adult subjects (79).
9 Intravenous Nicotine Self-Administration in the Rat: Extended Access Procedures
The widely used Corrigall–Coen procedure permits limited, 1-h daily access to nicotine. However, some researchers provide more extended access. For example, Sharp and associates permitted rats to lever-press for infusions of nicotine over a 23-h period (51). This procedure has better face validity, and provides nicotine plasma levels closer to those found in human smokers (80). Interestingly, total daily nicotine intake in the unlimited access procedure is not significantly different than in the limited access procedure. Extended-access nicotine IVSA has been studied with respect to strain (57), age (81), extinction behavior (55) and pregnancy (70). Somewhat disheartening, from a treatment standpoint, is the finding that reduction in access to nicotine results in a compensatory increase in daily intake that rebounds above baseline levels upon resumption of normal access (68).
10 Drug Treatments that Reduce Intravenous Nicotine Self-Administration in Rodents
Many drugs have been tested in rodent nicotine IVSA procedures, providing some insight into the neurobiological mechanisms underpinning this behavior. Typically, drug challenges are presented acutely to rats that have already acquired nicotine IVSA in limited-access sessions on a fixed-ratio schedule of reinforcement. This behavioral assay is also widely used to screen for novel drugs that might inhibit cigarette smoking, although the predictive validity of this procedure has not been established (26, 38). Here, we highlight some of the main findings in the drug-testing arena; more detailed reviews are available elsewhere (82–84). Unless otherwise stated, studies refer to acute drug effects on established nicotine IVSA.
10.1 Nicotinic Treatments
All first-line smoking cessation drugs (nicotine, varenicline, bupropion) act on nicotinic acetylcholine receptors (nAChRs). The effects of nicotine replacement therapy have been modeled in rats receiving chronic passive subcutaneous or intravenous infusion of nicotine (80, 85). Nicotine IVSA was markedly reduced, but it is not clear whether the passive nicotine regimen was effective: (1) by substituting for the reinforcing effect of self-administered nicotine, (2) by inhibiting the reinforcing effect of self-administer nicotine (e.g., by inducing nAChR desensitization), or (3) whether it allowed an aversive threshold to be reached more readily.
Nicotinic receptor antagonists have been extensively evaluated in the IVSA paradigm. The broad-spectrum nAChR blocker mecamylamine reduces nicotine self-administration reliably when administered acutely to rats (73, 86–89) and mice (5, 90); mecamylamine has even been used as a positive control substance for drug screening purposes (91–93). With several self-administered drugs, operant responding transiently increases when saline is substituted. In rats trained to self-administer intravenous nicotine, only a small “extinction-like” burst of responding has been observed upon saline substitution (55), and none after acute mecamylamine challenge (49, 55, 65) (see Fig. 2).
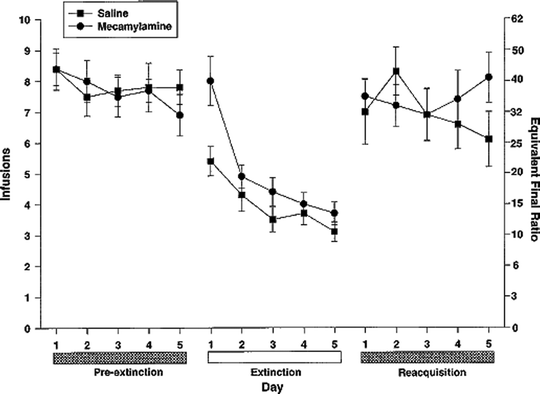

Fig. 2.
Effects of saline substitution and mecamylamine (nicotinic antagonist) challenge on intravenous nicotine self-administration. Adult rats were first trained to lever-press for infusions of 0.06 mg/kg on a progressive ratio (PR) schedule of reinforcement, in daily 1-h sessions. After a pre-extinction baseline period, each rat was tested under two conditions for five consecutive days, in a counterbalanced order: after saline-for-nicotine substitution, and after acute challenge with mecamylamine with nicotine still available. Both conditions led to an extinction-like decrement in responding. Data are mean ± SEM (Taken from (73). With kind permission).
A number of other nAChR antagonists have also been shown to reduce intravenous nicotine self-administration. These include: dihydro-beta-erythroidine (DHβE), considered to be a β2*-selective antagonist (92, 93); methyllycaconitine (MLA), a poorly selective α7 nAChR antagonist (94); the α3β4 antagonist 18-methoxycoronaridine (18-MC) (87, 95); N, N’-dodecyl-bis-picolinium dibromide (bPiDDB) (96); and bupropion (see below). Chlorisondamine, which blocks central nAChRs for many weeks after a single administration (97), produced a successive decline in nicotine self-administration in the days following antagonist administration (24). Intravenous nicotine self-administration is also inhibited by negative allosteric modulators of nAChR (98), the nAChR agonists SSR591813 (99) and isoarecolone (100), and by nAChR partial agonists including varenicline (91, 101). It is perhaps surprising that nicotine and nicotinic antagonists both reduce nicotine IVSA; conceivably, acute challenge with nicotine produces significant desensitization at nAChRs that are critical to IVSA (102).
The atypical antidepressant bupropion (Welbutrin™, Zyban™) is a moderately effective smoking cessation aid (103). However, in rodent studies of nicotine self-administration, bupropion has met with mixed results: acute and chronic treatments have been shown to reduce (87, 104–106), have no effect on (107), or increase responding for nicotine (105, 108). Response inhibition reliably occurs after acute high doses of bupropion (38, 105), but this is behaviorally nonspecific, insofar as high doses also attenuate responding for sucrose (104, 105) and food (108). Bupropion possesses some antagonistic activity at nAChRs (109), although behaviorally effective doses do not appear sufficient to inhibit nAChRs in rats (110); the drug can also inhibit plasmalemmal monoamine transporters (110). However, the mechanisms underlying bupropion’s therapeutic action remain obscure, and pharmacological analysis of bupropion’s behavioral effects is complicated by the formation of long-lasting active metabolites and also by species differences in metabolism (111, 112).
Lastly, active immunization against nicotine reduced drug intake substantially in rats (113), and nicotine vaccines are currently in clinical trials.
10.2 Dopaminergic Treatments
Dopamine (DA) has received a great deal of attention as a putative “reward” transmitter. Extracellular DA levels are increased during nicotine self-administration in limited-access sessions (114), but the effects of DAergic drugs on nicotine IVSA have been little studied. Corrigall and Coen demonstrated that the DA D2 receptor antagonists haloperidol and spiperone, as well as the D1 receptor antagonist SCH 23390, reduced nicotine intake; however, these drugs also reduced locomotor activity. Furthermore, SCH 23390 also reduced food self-administration (115), suggesting a generalized behavioral suppression. More recently, a DA D3 receptor antagonist SB277011A was shown to reduce nicotine IVSA, while sparing food-reinforced responding (116); a high dose was required, suggesting that D3 receptors may not have been responsible. Nicotine IVSA is also affected by acute challenge with the DA agonists apomorphine (105) and methylphenidate (117).
10.3 Glutamatergic Treatments
Glutamate (Glu) is the primary excitatory neurotransmitter in the brain and it provides some promising targets for addiction treatments. For example, chronic treatment with N-acetylcysteine, a nutritional supplement that increases cystine–glutamate exchange, was recently shown to reduce cigarette smoking (118). Initial studies in rats showed that the NMDA receptor antagonist dextromethorphan reduced nicotine self-administration (87, 119), but also self-administration of water (119). Antagonists targeting the mGluR5 glutamate receptor subtype have received considerable attention, particularly 2-methyl-6-(phenylethynyl) pyridine (i.e., MPEP). MPEP and its more selective analog MTEP consistently reduced nicotine self-administration in rats with little or no effect on food-reinforced responding (120–124). Agonists and antagonists of mGluR2/3 receptors have also been reported to reduce nicotine self-administration (see Fig. 3), when given either alone (LY379268) (125) or in combination (LY341495) with MPEP (122).
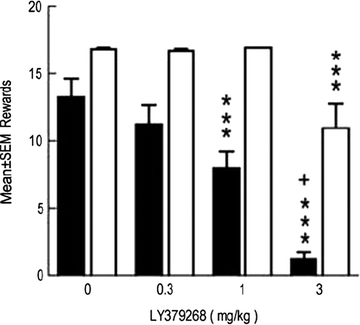

Fig. 3.
Effects of the metabotropic glutamate (mGlu) 2/3 receptor agonist LY379268 on nicotine-and food-reinforced responding. Adult rats were trained to lever-press for infusions of 0.03 mg/kg nicotine or food pellets, both on a FR5 schedule of reinforcement, in daily 1-h sessions. Acute systemic administration of LY379268 decreased nicotine self-administration (black bars) with less effect on food-reinforced responding (white bars). Data are mean ± SEM (Taken from (125). With kind permission).
10.4 Gamma Aminobutyric Acid (GABA) Treatments
The GABAB receptor agonist baclofen exerts acute biphasic effects on intravenous nicotine self-administration, with low doses increasing responding and high doses decreasing responding for nicotine (126). Both GABAB agonists and newer GABAB positive allosteric modulators reduce nicotine self-administration, with the latter showing useful selectivity for nicotine- versus food-reinforced responding (127–129).
10.5 Noradrenergic and Serotonergic Treatments
There is little published information about noradrenergic and serotonergic drugs, and results are not promising as regards smoking cessation. Reboxetine, which potently inhibits certain nAChRs as well as the norepinephrine transporter (NET), reduced self-administration of nicotine, but also inhibited sucrose self-administration (86). Similarly, the NET blocker desipramine inhibited both nicotine and food self-administration (130). Serotonergic (5-HT) antagonists have not been systematically studied, although in one report 5-HT3 receptor blockers were ineffective in reducing nicotine intake (131). In squirrel monkeys, the 5-HT transporter blocker sertraline failed to affect established nicotine IVSA (132).
10.6 Opioid Treatments
There is evidence both for and against opioid receptor involvement in nicotine’s rewarding effects (133). For example, nicotine conditioned place preference was found to be absent in mu-opioid knockout mice (134) and in wild type animals, the expression of place preference was abolished by systemic administration of the opioid antagonist naloxone (135). In contrast, the opioid antagonists naltrexone and naloxone had no effect on intravenous nicotine self-administration, despite reducing cocaine self-administration (48, 136). A recent, more detailed analysis suggests that naltrexone reduces the incentive properties of nicotine-associated sensory cues without reducing the primary reinforcing effects of the drug (133). However, human studies have revealed mixed effects of naltrexone on cue-induced craving and smoking behavior (137).
10.7 Cannabinoid Treatments
In animal studies, CB1 receptor antagonists have been shown to reduce self-administration of several drugs of abuse, including nicotine. However, the effect of CB1 receptor antagonists may be partially nonspecific, in that SR141716 (i.e., rimonabant) reduced inactive lever pressing (138) and AM251 inhibited responding for food reward (139). As discussed elsewhere, CB1 antagonists appear to inhibit IVSA at least partly by attenuating the impact of nicotine-associated cues (140, 141).
10.8 Brain Lesions
Several types of anatomical lesions have been applied to nicotine IVSA in rats. In particular, 6-hydroxydopamine lesions of the nucleus accumbens (NAcc) markedly attenuated this behavior (24), consistent with a role for mesolimbic dopamine in mediating nicotine reward or some related motivational process (Fig. 4). Excitotoxic lesions of pedunculopontine tegmental nucleus (PPTg) also reduced established nicotine self-administration behavior (142), while lesions of the posterior (but not anterior) PPTg (143), and neonatal frontal cortex (144) reduced acquisition of nicotine self-administration.
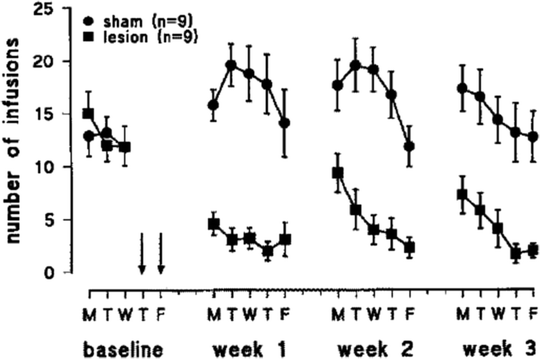

Fig. 4.
Effects of 6-hydroxydopamine lesions of the mesolimbic DA system on nicotine self-administration. Adult rats were trained to lever-press for infusions of 0.03 mg/kg nicotine on a FR5 schedule of reinforcement in daily 1-h sessions. The rats subsequently received bilateral intra-accumbens infusion of the dopamine-depleting neurotoxin or vehicle. After recovery from surgery, nicotine self-administration was found to be profoundly reduced. The same animals were tested for food-reinforced responding on Saturday and Sundays between weeks 1–2 and 2–3, which may account for the boost in intravenous self-administration (IVSA) early in weeks 2 and 3. Data are mean ± SEM (Taken from (24). With kind permission).
10.9 Central Drug Administration
Intracerebral administration of agonists or antagonists in rats has provided information about potential mechanisms underlying nicotine IVSA behavior. Three brain areas have been targeted: the NAc, ventral tegmental area (VTA), and PPTg. Intra-NAc administration of the mGluR2/3 agonist LY379268 inhibited nicotine self-administration (125), whereas focal infusion of the nicotinic antagonist DHβE did not (145). Intra-VTA administration of several drugs – notably DHβE (145), LY379268 (125), muscimol, baclofen, and the mu opioid receptor agonist DAMGO (146) inhibited nicotine IVSA. Finally, DHβE (142) and GABAB agonists also reduced nicotine self-administration when they were given into the PPTg (147).
10.10 Knockouts and Transgenics
Nicotinic receptor (nAChR) subtypes are defined primarily by subunit composition, and are highly heterogeneous. Knockout and transgenic mice have implicated a subset of nAChR subunits in nicotine IVSA, as reviewed previously (8, 102). Nicotinic AChR deletion can produce behaviorally selective effects, shown by the finding that β2 mutant mice self-administered cocaine but not nicotine (148, 149). Recent studies have combined two experimental strategies: genetic knockout, followed by intracerebral injection of a viral vector in order to express the deleted nAChR subunit within a brain structure of interest. This approach has identified α4β2- and α6β2-containing nAChRs in the VTA as critical to nicotine IVSA in the mouse (150).
11 Drug Treatments that Reduce Relapse to Nicotine Seeking
Relapse is a recurring problem in the treatment of smoking. Indeed, of smokers who quit without pharmacological or psychological support, only 3–5% remain abstinent after 6–12 months (151). In abstinent subjects, drug seeking can be triggered by a variety of stimuli including drug-associated cues, exposure to the drug itself, or stress. Different relapse scenarios are modeled in the rodent reinstatement procedure of drug self-administration, pioneered by de Wit and Stewart (152). In this procedure, rats are first trained to self-administer a drug. Self-administration behavior is then extinguished over multiple sessions, during which drug-associated cues may or may not be available. Animals are then acutely exposed to three types of stimuli: the drug itself, stressors, or drug-associated cues provided the latter have not been already extinguished. The resultant increase in (unreinforced) responding provides a measure of drug seeking. Brain mechanisms underlying reinstatement are highly dependent on the nature of the triggering stimulus (153). This behavioral procedure is now widely used to screen drugs for therapeutic potential.
Reinstatement related to nicotine IVSA has recently been reviewed in detail (9). Reinstatement has been induced by intravenous and systemic injections of nicotine (154, 155), footshock stress (156) and re-exposure to nicotine-associated stimuli (157). Nicotine-induced reinstatement was inhibited by antagonists of dopaminergic (SB277011A), glutamatergic (MPEP, EMQMCM) (158, 159), and cannabinoid (AM251) (139) receptors; the mGluR1 antagonist EMQMCM also reduced reinstatement of food seeking (159). In contrast, stress-induced reinstatement was attenuated by both the corticotropin-releasing factor (CRF) receptor 1/2 antagonist D-Phe CRF (12–41) (administered icv) and the α2 adrenergic receptor agonist clonidine (administered systemically) (160).
Studies of cue reactivity in cigarette smokers have largely focused on craving and physiological measures rather than drug seeking behavior itself (161). Indeed, there is surprisingly little direct evidence that smoking cues actually promote the occurrence of smoking behavior (161–163). Nevertheless, various drugs have been screened in animals for their ability to block reinstatement of nicotine seeking triggered by nicotine-associated cues. Drugs that inhibit reinstatement include the nAChR antagonist mecamylamine (164, 165), and modulators of GABA (CGP44532) (127), glutamate (MPEP, EMQMCM) (159, 166) and cannabinoid receptors (SR141716, AM251) (139, 167). In contrast, bupropion inhibited nicotine self-administration prior to extinction, but acutely increased reinstatement triggered by nicotine-associated cues (157). Speculatively, the latter result may be related to the limited efficacy of bupropion in smoking cessation (but see above).
12 Miscellaneous Issues and Recent Developments
12.1 Reinforcement Enhancement by Nicotine
Through conditioning, stimuli associated with smoking can elicit effects in their own right (168). For example, initially neutral cue complexes (visual, auditory and olfactory components) can elicit high levels of cigarette craving after they have been repeatedly paired with smoking (169). With the average smoker taking thousands of puffs a year, the extensive range of stimuli that can become associated with smoking are significant hurdles for cessation treatment. In human smokers, individuals who relapse tend to react more strongly to smoking-related cues than those who remain abstinent, and reactivity to these cues has some predictive validity in terms of successful quitting (170).
Caggiula and colleagues have recently shed new light on behavioral processes maintaining nicotine IVSA in the Corrigall–Coen model (171). Specifically, these investigators have systematically shown that intravenous nicotine exerts only weak primary reinforcing effects, and instead maintains self-administration behavior mainly by a process which they term “reinforcement enhancement.” The main evidence for reinforcement enhancement is as follows. Nicotine IVSA procedures incorporate a visual (or auditory) stimulus that is paired with each nicotine infusion, and nicotine IVSA is dependent on the presence of this stimulus (88, 92, 172–179). However, such stimuli tend to be reinforcing in their own right. For example, rats will typically press a lever in order to briefly illuminate a light stimulus, even when this is never paired with nicotine (171, 180, 181). Critically, responding for a visual stimulus can be enhanced by non-contingent administration of nicotine (see Fig. 5), delivered either via yoked passive infusions (173, 174, 179) or by continuous infusion (173). This reinforcement-enhancing effect of nicotine has been seen on most schedules of reinforcement (but not on FR1 (182)) and across a wide dose range (174).
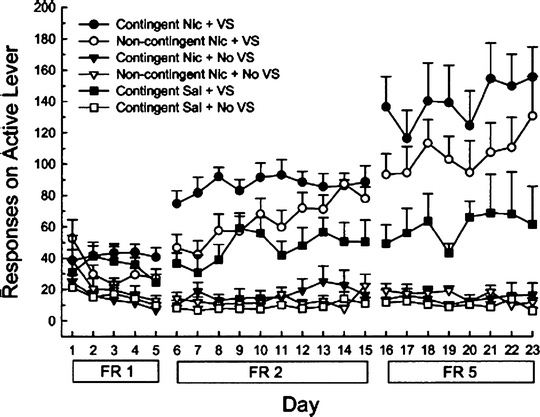

Fig. 5.
Reinforcement enhancing effect of nicotine. In this experiment, operant responding was tested with three successive schedules of reinforcement (FR1, FR2, and FR5) in daily 1-h sessions. Four (“contingent”) groups of rats were permitted to lever-press for intravenous infusions (0.03 mg/kg nicotine or saline), with or without accompanying visual stimuli (VS). Two other groups were yoked to self-administering rats and received intravenous nicotine non-contingently; one of these groups was permitted to respond in order to obtain visual stimuli. When available alone, nicotine was not significantly self-administered, whereas the visual stimuli were somewhat reinforcing. Both self-administered and non-contingent nicotine rendered the visual stimuli more reinforcing. Data are mean ± SEM (Taken from (173). With kind permission).
12.2 Possible Interactions between Nicotine and Other Smoke Constituents
Interactions between nicotine and environmental stimuli suggest one mechanism by which the drug could help to maintain cigarette smoking. Another widely discussed possibility is that the reinforcing effects of nicotine in smokers are potentiated by other smoke constituents, particularly monoamine oxidase (MAO) inhibitors. The basis for this notion is as follows. Cigarette smokers possess lower levels of brain and peripheral MAO compared to non-smokers and ex-smokers, with reductions in both MAO-A and MAO-B isoforms (183, 184). Cigarette smoke contains several identified MAO inhibitors (185). In rats, MAO inhibition results in increased extracellular DA in the NAc; whereas, local destruction of DA terminals is associated with a profound reduction in nicotine IVSA (24). Hence, tobacco-associated MAO inhibitors may potentially augment DA-dependent reinforcing effects of nicotine (or perhaps promote cigarette smoking by some other mechanism).
Several MAO inhibitors have been tested for their effects on nicotine IVSA in rats. Published work in this area has originated from two groups. Stinus and associates administered several MAO inhibitors (tranylcypromine, phenelzine, clorgyline and norharman) on a daily basis during acquisition of fixed-ratio schedule responding and after transfer to a partial reinforcement schedule (186, 187). MAO inhibitor treatment selectively increased responding on the PR schedule (Fig. 6). These findings suggest that chronic MAOI treatment did not enhance learning, and may instead have increased the rats’ motivation to self-administer nicotine (an aspect thought to be most directly measured by PR schedule responding). It is also possible, however, that the effects of these MAO inhibitors took time to emerge or required chronic administration in order to develop. In contrast, Leslie’s group found that daily MAO inhibitor pretreatment (tranylcypromine) did enhance acquisition of nicotine IVSA (188–190), an effect that was blocked via the α1-adrenergic receptor antagonist prazosin (189).
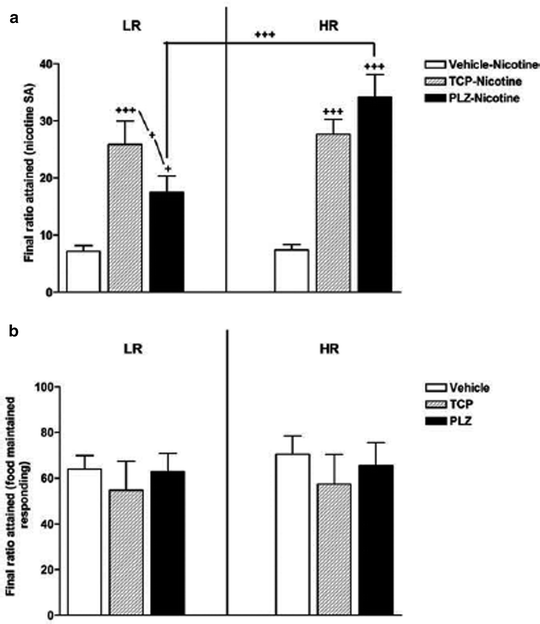

Fig. 6.
Stimulation of nicotine IVSA by daily administration of MAO inhibitors. Rats were initially screened for responsiveness to novelty, and allocated to low- and high-responder groups (i.e., LR and HR). Subjects then learned to self-administer nicotine (0.03 mg/kg i.v.) on several schedules of reinforcement, culminating in PR (shown here). Throughout the self-administration experiment, each rat received tranylcypromine (TCP, 1.5 mg/kg/day, i.p.), phenelzine (PLZ, 2 mg/kg/day, i.p.), or vehicle. The y-axis shows the final ratio attained in order to obtain nicotine infusions (upper panel) or food pellets (lower panel). According to this measure, both monoamine oxidase (MAO) inhibitors greatly increased the motivation to self-administer nicotine but not food. Data are mean ± SEM (Taken from (186). With kind permission).
These findings are certainly of interest but require careful interpretation. First, all published animal studies employed high doses of MAO inhibitors, resulting in a near-total inhibition of enzyme activity. In contrast, MAO-A and -B isoforms are only inhibited by about 30–40% in human brain (183, 184). Second, animal studies have largely relied on frequent (i.e., daily) administration of irreversible MAO inhibitors, even though MAO activity returns only slowly after irreversible inhibition, with a recovery half-life exceeding one week in rats (191). Use of repeated high doses is of particular concern since MAO inhibitors exert numerous off-target actions. Third, in some studies (188–190) nicotine IVSA itself occurred only in the presence of MAO inhibition. The latter studies examined only the first 5 days of IVSA training, and it would be interesting to know whether the enhancement would persist with further testing.
Aside from MAO inhibitors, only two other tobacco smoke constituents have been examined in the nicotine IVSA paradigm: acetaldehyde and nornicotine. Acetaldehyde, at a dose that was not itself self-administered, increased nicotine self-administration in adolescent rats; however, this facilitatory effect was not observed in adults (192). Furthermore, the locomotor stimulatory effect of nicotine was also increased by pretreatment with acetaldehyde in both adolescents and adult rats (193), suggesting that generalized activity may be partly responsible for the increase in IVSA. These findings clearly merit further study.
Nornicotine is a nicotinic agonist that is present in tobacco smoke and also formed from nicotine metabolism. It has a longer elimination half-life than nicotine, and may reach pharmacologically significant concentrations in cigarette smokers (194). Rats have been found to self-administer a range of nornicotine doses (peak responding at approximately 300 μg/kg/infusion), showing normal extinction and reinstatement upon removal and replacement of the drug (195). It should be noted, however, that nornicotine is less prevalent than nicotine in cigarette smoke, and much of it is formed in vivo from nicotine. Hence, the pharmacological impact of nornicotine may be slowed by the need for nicotine metabolism.
Collectively, the findings reviewed in this section suggest that some non-nicotine tobacco constituents may enhance the reinforcing effect of nicotine. Tobacco smoke may also contain chemical reinforcers other than nicotine. Although only one such candidate (nornicotine) has come to light so far, it is worth bearing in mind that tobacco addiction, from a pharmacological point of view, may constitute more than just an addiction to nicotine.
12.3 Nicotine Self-Administration in Relation to Stress
Several studies have described the effects of chronic nicotine self-administration on stress-related processes in adult rats. For example, rats given prolonged (e.g., 40 days) access to nicotine not only displayed higher levels of social anxiety (196) but also mounted exaggerated adrenocorticotropic hormone (ACTH) and corticosterone (CORT) responses to a mild stressor (197). Chronic nicotine self-administration, similar to chronic stress (198), also increased CRF mRNA in the paraventricular nucleus (PVN) of the hypothalamus (199). Furthermore, chronic nicotine increased NMDA and glutamate (Glu) receptor prevalence in the medial prefrontal cortex (mPFC) and VTA respectively (200), as well as Glu release in these areas (201) – another effect common to chronic stress (202). Somewhat surprisingly, however, norepinephrine (NE) release in the amygdala was enhanced only during acquisition of nicotine self-administration, but normalized over time (203), suggesting tolerance.
12.4 Other Consequences Associated with Nicotine Self-Administration
Numerous neuropharmacological and behavioral changes have been reported in rats self-administering nicotine, and the following overview is not intended to be exhaustive. Some aspects are reviewed in more detail elsewhere (82, 204, 205). Although the focus has been on changes occurring in the CNS, chronic nicotine exposure and/or IVSA can also lead to various peripheral changes, such as in T-cell responsiveness (206).
The immediate early gene product Fos is commonly used as a marker of neuronal activation. Intravenous nicotine increases Fos expression in a variety of brain areas, both after passive infusions (207, 208) and self-administration (207, 209) in rats. In one IVSA study, Fos expression was increased in 43 of 77 brain areas examined, including the mPFC, NAc shell (NAcSh), and cingulate cortex (209). However, it is not clear whether this Fos induction was related to the reinforcing effects of nicotine, since the experimental design did not include a control group receiving passively administered nicotine. A passive nicotine control was included in a subsequent study (207), but this group was not matched for prior nicotine exposure. Acute passive administration of intravenous nicotine also increased glucose utilization, another marker of neuronal activity, but unlike Fos induction, this effect was apparently restricted to NAcSh (210).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


