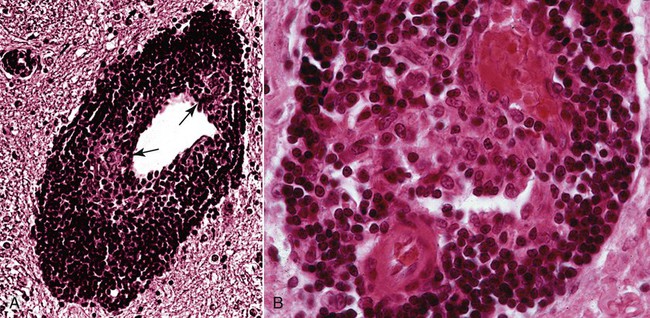
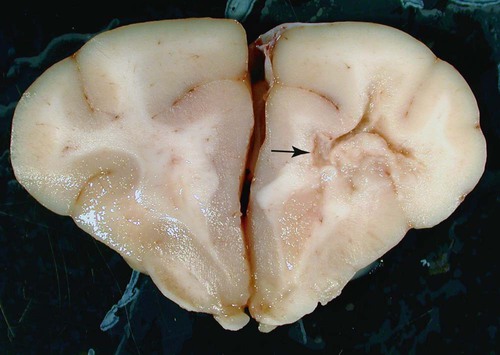
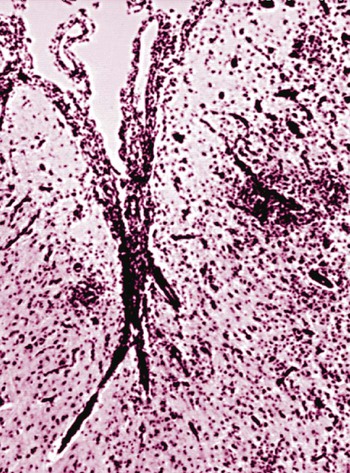
The conditions described in this chapter are suspected to be caused by insidious infectious agents or immune aberrations in response to one or more stealth pathogens. The central nervous system (CNS) is a frequent site of localization of pathogens evading host immune responses. Prion diseases are included here, partly on a historical basis because they eluded detection for many years. Furthermore, their place as a true infectious disease exists outside the realm of the classical infectious agents. In addition to the conditions described in this chapter, other CNS diseases of suspected infectious origin are occasionally encountered in dogs and cats during diagnostic work-ups. Some will remain a single sporadic observation; others will become recognized as disease entities. The ability to characterize infectious agents in these diseases is limited when formalin-fixed tissues are used. Fresh material for culture should be collected when clinical findings are compatible with one of these disorders. In addition to brain tissue taken for isolation procedures, serum and cerebrospinal fluid (CSF) should be collected and screened for antibody activity against a wide variety of known infectious agents. The advent of widely available, extremely powerful molecular biologic techniques also considerably extends the range of etiologic studies. In addition, more systematic ultrastructural studies of appropriately fixed tissues taken from lesions and sites indicated by the clinical examination may be helpful in detecting infectious agents. For a discussion of bacterial CNS disorders, see Bacterial Infections of the Central Nervous System, Chapter 91. Andrea Tipold, Marc Vandevelde, and Scott J. Schatzberg Granulomatous meningoencephalitis (GME) has been reported in dogs in the United States,24,25,25 Australia,66 New Zealand,5 Japan,104 and several European countries.200 It is a relatively common condition and is an angiocentric, nonsuppurative, mixed lymphoid inflammatory process, predominately affecting the white matter of the brain and spinal cord.25,41 Despite its recognition as a clinical entity for over 40 years, the underlying cause and mechanism of disease production remains unclear. Genetic, autoimmune, infectious, neoplastic, and even toxic causes have been theorized. Previous work has demonstrated that females are predisposed to GME; this finding is similar to other autoimmune demyelinating diseases including multiple sclerosis and experimental autoimmune encephalitis.79,92,92 The explanation of the female predisposition for autoimmune CNS diseases is unclear; however, a connection between sex steroid–associated alterations in T-helper cytokines, suppression of regulatory cytokines, and X-chromosome susceptibility alleles may be involved.79,92 GME has been proposed by some to be a delayed-type hypersensitivity reaction with an autoimmune basis, supported by a predominance of major histocompatibility complex class 2 and CD3+ T cells.101 Other investigators have subsequently confirmed a predominance of CD3+ T lymphocytes and a complete absence of the CD79 (B-cell marker) immunoreactivity in four cases of GME.182 However, these investigators were unable to demonstrate statistical differences in the number of CD3+ cells between GME and necrotizing meningoencephalitis (NME) or GME and central malignant histiocytosis.181,182 Results of preliminary immunophenotyping studies indicate a consistent pattern among disseminated GME cases.83 Anti-astrocytic antibodies also have been identified in the CSF of dogs with GME.129 The complete immunologic profile of GME and whether CNS autoantibodies are the cause or consequence of inflammation remain to be elucidated. Despite the conventional view that GME is a disorder of immune dysregulation, some veterinary neuropathologists suggest that GME is a lymphoproliferative disorder, with features of both inflammation and neoplasia.180 Focal GME is particularly similar to neoplasia, because lymphocytes within the perivascular cuffs often have variable degrees of pleomorphism and mitotic indices.57 Interestingly, CSF from disseminated GME cases occasionally contains lymphoblasts.160 It is unclear whether the abnormal lymphocytes within brain lesions or CSF are reactive inflammatory cells or representative of a true neoplastic population. Potential infectious triggers for GME have also been considered and investigated.159,161 Borna disease virus was reported in several dogs with meningoencephalitis in Japan and Switzerland and has been proposed as a causative agent for GME.137,211 Borna disease virus, however, is unlikely to be a common etiology for GME, given its predilection for CNS gray matter. Other investigators163 have demonstrated positive immunohistochemistry for West Nile, canine parainfluenza, and encephalomyocarditis viruses in severe lesions of dogs with meningoencephalitides of presumed unknown origin. The significance of these observations is unclear, because the positive immunohistochemistry may be caused by antibody cross-reactivity with endogenous proteins, as has been described with measles infections.168 Alternatively, these results may support the theory that GME is a nonspecific hyperinflammatory response of the host to various antigens, of which pathogens comprise an important subset. To date, molecular investigations led by one of the authors of this chapter (SJS) have failed to disclose consistent infectious agents associated with GME; however, work is ongoing to investigate an extremely diverse group of potential viral, bacterial, and rickettsial triggers.159 Further investigation has identified Bartonella spp. and Mycoplasma spp. in sporadic, confirmed cases of disseminated GME.11 GME is most likely a nonspecific immunologic response, and multiple environmental triggers (pathogens, vaccines) and genetic factors are likely to play roles in the etiopathogenesis. The disease occurs most often in young to middle-aged small purebred females, but dogs of different ages, breeds, and sex can develop the disease. The prevalence for smaller breeds, especially poodles, small terriers, Pekingese, and Maltese, appears to be higher.24 The disease rarely occurs before the age of 2 years. Clinical signs usually have an acute onset, and the disease typically is progressive. In acute GME, clinical signs develop rapidly over the course of 1 to 10 days, and without treatment, it is rapidly progressive and fatal. Clinical signs also may develop more slowly over a period of 1 to 4 months. Characteristic neurologic signs with GME cannot be expected because lesions can occur anywhere in the CNS. Signs are associated with the site of most extensive damage, or may reflect a multifocal lesion. Signs consist of disturbances in gait and postural reactions, with ataxia, dysmetria, paresis, and paralysis, and changes in mental status, with confusion, lethargy, and coma. Cranial nerve dysfunction may also be observed. Despite the nonspecific features of the CNS signs, some predictable findings may be observed. In the acute stage of the disease, more than 50% of animals have fever, and many exhibit paraspinal discomfort on palpation174 or signs of cervical pain.41,175 In this stage of the disease, central vestibular signs are common (Fig. 82-1); seizures also may be observed. The clinical presentation alternatively may suggest a space-occupying mass referring to a single site of involvement, most frequently the posterior fossa, with brainstem and cerebellar signs. Cerebral or spinal signs sometimes predominate. A particular ocular form of GME, previously called ocular reticulosis, occurs with acute unilateral or bilateral blindness and ophthalmoscopic evidence of optic neuritis.24,103 Typical hematologic and biochemical findings are not observed. Neutrophilia may be present in the acute phase of disease.174,175 The CSF analysis is abnormal, with a marked pleocytosis; cell counts of 100 to several thousand leukocytes per microliter are usual. Mild pleocytosis or even a normal cell count is observed in animals pretreated with glucocorticoids. Mononuclear cells predominate, but a mixed pleocytosis can also be observed with varying numbers of neutrophils.10 Cytologic examination of the CSF may reveal large anaplastic mononuclear cells with abundant lacy cytoplasm,24 and up to 20% are macrophages. Protein concentration usually is increased, with a range from reference values up to 1119 mg/dL and a mean of 163.2 ± 25 mg/dL.10 Electrophoretic examination of the CSF may suggest blood-brain barrier disruption and intrathecal immunoglobulin synthesis.174,175 Such changes are not specific for GME. Electroencephalographic patterns studied in some dogs with GME were nonspecific. In the rare focal form of GME, the tumorlike lesions can be detected with computed tomography (CT)26 after intravenous inoculation of contrast medium and magnetic resonance imaging (MRI).131 With CT, GME lesions are contrast enhanced, though not to the degree of meningiomas.176 MRI features can resemble neoplasia in which lesions with T1-weighted images appear isointense and have hypointense margins, but lesions with T2-weighted images appear hyperintense.115 Inflammatory lesions may have lesions on T1– and T2-weighted images that enhance with contrast.115 Regardless of ancillary testing, a definitive antemortem diagnosis requires histopathologic examination of brain biopsies. These specimens must be taken by stereotaxic equipment, surgical biopsy, or necropsy. Without these diagnostic modalities, GME can be mistaken for a neoplasm or another focal encephalitis.182 Mixed cell patterns and increased proteins in CSF also may be present with inflammatory encephalitis produced by canine distemper virus, Neospora caninum, Toxoplasma gondii, mycoses, and various other CNS infections. Special stains can be applied to specimens, and molecular genetic tests may be performed on CNS tissue to help determine potential agents causing the lesions. Polymerase chain reaction methods (PCR) can be used on CSF or CNS specimens in an attempt to determine an underlying infectious cause of GME. The use of these procedures has been reviewed elsewhere.11,159,161,163 Particular chapters dealing with the respective infectious agents under consideration should be consulted. Lesions are found in the meninges, brain, and spinal cord, especially in the cervical segments. White matter often is more severely affected compared with gray matter. Although the lesions are disseminated, the cerebral hemispheres, the midbrain, and the region around the fourth ventricle are most frequently involved. The lesions, which are strictly associated with the vasculature, consist of perivascular cuffing of monocytes, lymphocytes, plasma cells, and sometimes a few neutrophils and mast cells. The formation of eccentrically situated nodular foci of macrophages within these cuffs is a characteristic finding in GME (Fig. 82-2). The cuffs can be distinguished in three degrees of severity: small disseminated perivascular lesions, confluence of several perivascular cuffs into small granulomas, and large focal coalescing granulomas, leading to the formation of tumorlike lesions. Additionally, necrosis of the neuropil, mitosis in the perivascular cuffs, and edema may be observed in both forms of the disease. Tryptase-positive mast cells can be found in perivascular cuffs of the lesions of GME, in the meninges, and in the CNS parenchyma. Dogs with the acute disease have significantly more mast cells compared to dogs with chronic disease, suggesting that this cell population may contribute to the dynamics of the lesion with resultant CNS injury, and to the rapid clinical deterioration of dogs with GME.52 Anti-astrocyte antibodies are increased in the CSF of dogs with GME and NME but not with other inflammatory conditions of the CNS.129 Autoantibody was also detected in some dogs with brain tumors, but this may represent only a secondary response to a damaged blood-CNS barrier. No specific therapy for GME exists, although improvement frequently has been observed after administration of glucocorticoids or diverse immunosuppressive drugs (Table 82-1). Because a definitive antemortem diagnosis of GME is difficult to make, treatment studies have been cumbersome. Because glucocorticoid therapy provides only temporary improvement in many cases, other immunosuppressive drugs have been used. The use of procarbazine or cytosine arabinoside, two alternative antineoplastic drugs, may be considered.45,136 Procarbazine is a prodrug that forms numerous active cytotoxic metabolites after metabolism. This DNA-alkylating agent is an anticancer drug that has also been used in human transplant cases. It can be used alone or in combination to treat presumptive GME.45,223 TABLE 82-1 Recommended Dosages of Immunosuppressive Drugs for Neurologic Diseases of Unknown Origin B, Both dog and cat; D, dog; IM, intramuscular; IV, intravenous; PO, by mouth; SC, subcutaneous. aFor more information on all drugs, see the Drug Formulary in the Appendix. bDose per administration at specified interval. cDose is gradually tapered over 4 months to the lowest that is effective in maintaining remission. Rarely can drug therapy be discontinued. dAfter 30 days, every-other-day dosing is given. With no control, dose is increased to 50 mg/kg every other day and 25 mg/kg on alternate days. eGenerally, the 3-mg/kg dose is used for induction. Response to treatment and blood levels should be monitored to achieve efficacy and avoid nephrotoxic side effects. Drug levels should be checked 4 to 6 hours after last dose. Dose is usually reduced to every 24 hours for long-term maintenance.1 Procarbazine is well absorbed orally and may cause neurotoxicity, vomiting, or hepatic dysfunction. The patient should be monitored closely for cytotoxicity, and after 1 month of daily therapy, every-other-day dosing should be used. Cytosine arabinoside, a parenteral drug, is also effective, has few side effects, and is reasonably priced.45 Because of its parenteral route, it is used primarily for induction therapy in the hospital. Both drugs cause myelosuppression, and animals must be monitored for signs of toxicity. Leflunomide is a pyrimidine-synthesis inhibitor that suppresses activated T cells. It has been used as an alternative treatment because of side effects or lack of responsiveness when other drugs are used. Leflunomide can affect cell replication, causing leukopenia, diarrhea, or alopecia in humans. Mycophenolate mofetil has been used to treat dogs with various immune-mediated diseases such as pemphigus foliaceus and myasthenia gravis. It inhibits an enzyme in purine synthesis, which in turn suppresses lymphocyte function. It causes minimal myelosuppression. Mycophenolate mofetil is expensive; however, it has a low degree of toxicity, which is usually associated with vomiting or diarrhea. Ciclosporin has shown to be of benefit in treating dogs with localized presumptive GME.1 The response to ciclosporin alone or initially in combination with glucocorticoids achieved remission and halted progression of neurologic signs for up to 1 year of monitoring with continued daily administration of a maintenance dosage. Use of ciclosporin without glucocorticoids avoided many of the undesirable side effects. Nephrotoxicity of ciclosporin was not observed, and blood monitoring showed safe therapeutic blood levels of the drug. Excess shedding of hair, alopecia, and potential worsening of an existing urinary tract infection were the observed side effects. In addition, the use of lomustine has been reported.61 In a study using prednisolone alone, or in combination with lomustine, the response to both regimens was equivocal.60a Therefore, before using immunosuppressive drugs, infectious disease causes of meningoencephalitis should be ruled out; otherwise, an exacerbation of the infection might occur. Radiation therapy with cobalt or linear accelerator has been used to slow the progression of the disease.134 Dogs with focal lesions survive longer than do those with multifocal neurologic signs.134 Radiation injury of cranial tissues is an adverse effect; however, this may be an alternative to systemic immunosuppression caused by chemotherapy. Anticonvulsants may be needed to control seizures associated with inflammatory forebrain lesions in dogs with GME. Oral phenobarbital and potassium bromide are indicated for general use, and intravenous or rectal diazepam should be used in cases of status epilepticus. The prognosis of GME is generally guarded given that most dogs develop progressive neurologic signs despite treatment. Dogs with multifocal GME may have a rapid and fulminant course of illness over several weeks that may be unresponsive to anti-inflammatory or immunosuppressive therapy. Patients with more focal signs can have progressive illness over several months153; those with focal forebrain localization have the longest life spans.134 Andrea Tipold, Marc Vandevelde, and Scott J. Schatzberg NME and necrotizing leukoencephalitis (NLE) are CNS inflammatory disorders with similarly elusive etiopathogeneses to that of GME. Historically referred to as “pug dog encephalitis” and “necrotizing encephalitis of Yorkshire terriers” (Fig. 82-3) respectively, these idiopathic meningoencephalitides have now been reported in various toy breeds including the pug, Maltese, Chihuahua, Yorkshire terrier, Pekingese, West Highland white terrier, Boston terrier, Japanese spitz, and miniature pinscher.* To avoid confusion associated with the breed-specific terminology, these inflammatory disorders are best described with neuropathologic nomenclature reflective of the topographies of the brain lesions associated with each (e.g., NME and NLE). Because of the overlap in clinical signs and neuropathology, the all-encompassing term necrotizing encephalitis (NE) may be preferable on an antemortem basis and has been used as the heading for this section (see also later discussion). The suspected genetic basis and subsequent pathogenic mechanisms resulting in NME and NLE are poorly understood. Pedigree information on a large cohort of pug dogs is suggestive of a strong familial inheritance pattern for NME.69 While this genetic transmission data is not surprising, a simple mendelian inheritance pattern could not be demonstrated. The latter suggests that NME is a multifactorial disorder. A complex genetic and infectious pathogenesis has been demonstrated for acute necrotizing encephalopathy in children, which occurs in susceptible individuals in association with influenza and parainfluenza infections.135 Missense mutations in the nuclear pore gene RANBP2 have been identified as susceptibility alleles for familial and recurrent cases of acute necrotizing encephalopathy. A similar combination of genetics and infectious factors may be responsible for canine NE. Molecular studies on affected dogs are ongoing to assess for genetic susceptibility loci and screen for a diverse group of potential infectious triggers that may lead to immune dysregulation in NME and NLE. Although it is tempting to consider NME and NLE as distinct entities, these disorders may represent a spectrum of CNS injury with similar pathogeneses. Interestingly, neuropathologists have evaluated the brains from pugs, several Maltese terriers, and Chihuahuas with lesions that have been typical of NLE, rather than NME, which has been reported in these breeds.49,83 Conversely, NME has been histopathologically confirmed in a Yorkshire terrier.160,180 Experimental autoimmune encephalomyelitis models in rats may provide insights into such variations in lesion topographies within and among different dog breeds. Minor modifications of major histocompatibility complex haplotypes in rats with experimental autoimmune encephalitis results in different but reproducible patterns of brain inflammation after exposure to a myelin-oligodendrocyte-glycoprotein.179 Similarly, the histopathologic differences among the NEs may result from minor genetic differences among dog breeds, modifying genes, and/or variations in antigenic exposures. An autoimmune pathogenesis has been suggested for NME based on the presence of anti-astrocytic and glial fibrillary acid protein autoantibodies in CSF of affected dogs.129,170,170 However, similar antibody levels occur in the CSF of dogs with GME or brain tumors, and even some clinically healthy dogs.170,198 Whether glial fibrillary acid protein autoantibodies are the inciting cause of NME, representative of a breed-specific fragility of astrocytes, or the consequence of prolonged tissue destruction secondary to infectious disease, requires further investigation. Results of genomic analysis of pug dogs with NME showed the presence of specific alleles at a locus near the dog leukocyte antigen complex on CFA12.70 This strong dog leukocyte antigen class II association with NME is similar to the pathogenic mechanisms and gene associations found in human multiple sclerosis. Because of the neuropathologic similarities of NME to viral meningoencephalitides in other species, viral etiologies have been considered.161 The histopathologic lesions associated with canine NME are especially similar to those present in human herpesvirus meningoencephalitis.42,214 Because canine herpesvirus-1 may cause encephalitis in neonates,2,143 it is conceivable that NE is triggered by a recrudescence of a latent herpesvirus infection. Interestingly, a “herpes-like virus” was isolated from one pug dog with NME; however, the viral isolate was not retained.42 Further attempts at viral isolation from dogs with NME have been unsuccessful180,192,192; however, Mx proteins, interferon-induced GTPases associated with viral and inflammatory diseases, have been demonstrated in brain tissues from pug dogs with NME.148 Results of broadly reactive PCR assays for herpesviruses have been negative on a large number of paraffinized and freshly frozen NME-affected brains.69,161 Moreover, PCR screening of NME-affected brains for an extremely diverse group of DNA and RNA viruses has failed to identify viral nucleic acids.160 Although the lack of viral nucleic acids in NME-affected brains argues against a directly acting, neurotropic virus, these PCR data do not exclude the possibility of a viral trigger for NE via molecular mimicry.56,138,138 Another possibility is that a pathogen is present in the CNS (but at undetectable levels) in the presence of a self-perpetuating immune response, a phenomenon that has been described for flaviviral infections.108 Investigations are ongoing to pursue direct acting neuropathogens as well as para- and postinfectious pathogeneses for autoimmune inflammation in canine NE. The onset of neurologic signs associated with NME varies from 6 months to 7 years of age and most commonly occurs in young dogs, with a mean age of 29 months.42,113 In comparison, NLE typically manifests between 4 months and 10 years of age, with a mean age of onset of 4.5 years.111 Dogs with both NME and NLE commonly manifest cerebrothalamic signs due to the predominance of lesions in the prosencephalon; NLE also may cause mid to caudal brainstem signs.50 However, because of the multifocal nature of inflammatory disease, variations may occur with either disorder, and clinical signs are primarily reflective of the lesion locations. In general, the signs associated with NE typically are rapidly progressive and most commonly include seizures, depression, circling, vestibulocerebellar signs, visual deficits, and ultimately death. No uniquely characteristic hematologic or biochemical findings have been observed with NE. CSF analysis results are abnormal in most dogs, with a predominantly mononuclear pleocytosis (usually 90 to 600 cells/µL) and an increased protein concentration (usually 50 to 200 mg/dL)42,94; however, these are not specific, because other encephalitides are associated with similar CSF changes. The massive necrotizing lesions in the cerebrum can be detected with cross-sectional medical imaging methods such as CT or MRI.110,177 Typical MRI lesions associated with NME include asymmetric, multifocal prosencephalic lesions affecting the gray and white matter, with variable contrast enhancement on T1-weighted imaging.220 Loss of gray and white matter demarcation also may be discernible.60,220 Lesions appear hyperintense on T2-weighted images and isointense to slightly hypointense on T1-weighted images, with slight contrast enhancement. In NLE, multiple, asymmetric bilateral prosencephalic lesions mainly affecting the subcortical white matter have been described.208 The NLE lesions are hyperintense on T2-weighted and fluid-attenuated inversion recovery images and often include multiple cystic areas of necrosis. These lesions are hypointense or isointense on T1-weighted images, and contrast enhancement is variable. MRI findings may increase the clinician’s confidence in a presumptive diagnosis of NE. Positron emission tomography has been used on a limited basis in an attempt to distinguish NME from other inflammatory brain diseases.55b,99a Necrotizing lesions in the cerebrum may be visible on macroscopic examination. The histologic findings are unusual and highly typical for this disease: disseminated meningitis, choroiditis, and encephalitis, with greatest involvement in the cerebrum. The perivascular and meningeal inflammatory infiltrates consisting of lymphocytes, plasma cells, histiocytes, and macrophages have a strong tendency to invade the adjacent parenchyma. Extensive compact subpial and subventricular lesions are present and may extend deep into the underlying brain tissue, with unusually intense microglial proliferation and total destruction of the original tissue elements. In some areas, frank cerebrocortical necrosis occurs. The cerebral white matter also is severely affected, with intense perivascular cuffing and gliosis (Fig. 82-4). In some areas, leukomalacia with liquefaction and cavitation of the tissue is noted. Immunosuppressive drugs may be used, as described previously, for dogs with GME (see Table 82-1).67,184 Controlled studies, however, are lacking, and the prognosis with immunosuppression remains guarded. Glucocorticoid monotherapy does not alter the course of this NE in confirmed cases, and antiepileptic drugs may be ineffective in controlling seizures.48,76 However, results of one study indicated that use of antiepileptic drugs was associated with a prolonged survival time.113 Affected animals usually develop persistent seizures and progressive neurologic signs and do not usually live longer than 6 months to 1 year after the onset of clinical illness. Andrea Tipold and Marc Vandevelde Hydrocephalus is probably the most common developmental abnormality of the CNS in dogs and may be the result of congenital or acquired stenosis of the mesencephalic aqueduct, resulting in internal hydrocephalus.88 Acquired hydrocephalus is usually a result of infectious or neoplastic diseases that lead to obstruction of the CSF drainage pathways. A particular form of acquired hydrocephalus in young dogs is associated with severe periventricular inflammation.* In one puppy, inflammation likely closed drainage from the skull, causing external hydrocephalus.54 Some investigators proposed that such inflammatory changes could be the result of ischemia induced by rapidly rising intracranial pressure.219a Because of the severity of the inflammatory changes seen with hydrocephalus, an infectious cause has been suspected. On bacteriologic examination of brain tissue and CSF in two dogs with this disease, three different bacteria were isolated; however, these were thought to be contaminants.84,217 Staphylococci were isolated in another case.54 Possibly, the underlying disease might be caused by a virus, although no infectious agent was found on electron microscopy study of the periventricular tissues of two dogs.217 Canine parainfluenza virus has caused hydrocephalus in dogs after experimental inoculation (see Clinical Findings, Chapter 7).15,16 However, the clinical and pathologic findings in experimental parainfluenza-induced hydrocephalus are totally different from those of the condition described herein. Serum-neutralizing antibodies against canine parvovirus (CPV) were absent.217 No consistent hematologic or biochemical findings are associated with this form of hydrocephalus. CSF abnormalities consist of xanthochromia, increased protein concentration, and pleocytosis, including erythrocytes, mononuclear cells, and macrophages containing erythrocytes. Radiographic examination of the skull reveals abnormalities such as thinning of the cranial vault and homogeneous appearance of the intracranial contents. CT and MRI confirm the enlargement of the ventricles. However, these findings are indicative of hydrocephalus, regardless of its cause.88 The same can be said for the electroencephalographic findings indicative for hydrocephalus.88 Massive enlargement of the cerebral ventricles occurs, which are filled with cloudy, hemorrhagic CSF. The internal surface of the ventricles is focally roughened, with a brownish discoloration. A typical finding is the presence of large dissecting cavities, also called false diverticula, in the cerebral mantle (Fig. 82-5, A). These dissecting cavities communicate with the lateral ventricles. Histologically, severe inflammation with necrosis of the ependymal and subependymal tissues can be found.32,203 The lesions are always hemorrhagic. Hemosiderin-laden macrophages are found in old lesions. Perivascular cuffing with inflammatory cells occurs and, in acute lesions, diffuse infiltration with neutrophils and macrophages (Fig. 82-5, B, and C). Glial mesenchymal repair tissue is formed later in the course of the disease. By the time the condition becomes evident to the owner, severe damage has already taken place. In most cases, the disease is rapidly progressive, leading to severe neurologic impairment. However, in cases in which the condition stabilizes, conservative therapy with glucocorticoids or surgical treatment may be considered.88 Antibacterial therapy and a ventriculoperitoneal shunt was successful in treating a puppy with external hydrocephalus associated with staphylococcal infection.54 Prognosis remains cautious given that placed catheters may become obstructed by the inflammatory reaction. Andrea Tipold and Marc Vandevelde A novel breed-specific meningoencephalitis has been described in young greyhounds from three different kennels in southern Ireland.31 Frequently several littermates are affected. An infectious origin is suspected, but no inclusion bodies, fungi, or protozoal cysts were seen. Additional tests for common antigens failed to determine the cause of the disease. Results of gene expression studies suggested that viral infection or autoimmunity may be involved in the pathogenesis.68a Neurologic signs occurred with acute onset and include central vestibular signs with head tilt, ataxia, and recumbency. Additionally, circling and blindness were common findings. The animals were mentally dull, were dehydrated, and had lost weight. Hematologic and biochemical parameters reflected only dehydration. Diffuse encephalitis with perivascular, predominantly mononuclear, cuffing was described. Results of a search for a wide range of known infectious agents was negative.171a As with all parvoviruses, CPV requires infection with rapidly multiplying cells (see Chapter 8). Generally, the gastrointestinal tract and bone marrow are affected in postnatal dogs. The myocardium and CNS tissues may become damaged when infections occur prenatally or neonatally, due to the more rapid cell division in these tissues (see Neurologic Disease, under Clinical Findings in Chapter 8). CPV has been identified in the CNS of pups with such infections in association with little or no inflammatory changes in the CNS. Although viral antigens are less commonly demonstrated in CNS tissue, CPV DNA and messenger RNA have been found in high levels, suggesting active replication of the virus in central nervous tissues. A leukoencephalopathy associated with “shaker puppies” was found in two related litters of Cretan hound pups with evidence of CPV replication in their CNS.162 Clinical signs became apparent when the puppies began to ambulate at 2 to 3 weeks of age. The dogs were hospitalized and monitored, and neurologic signs were nonprogressive. Some of the affected pups had anorexia, vomiting, and hemorrhagic diarrhea, with a positive fecal antigen test result, during their first week of hospitalization. Histopathologic changes included mild lymphohistiocytic inflammatory changes with mild to moderate vacuolation with myelin loss predominately affecting the white matter of the cerebellum. Genetic factors and a superimposed viral infection may have been responsible for the development of neurologic signs; however, further studies will be needed because inherited leukoencephalopathies can occur independent of viral infections, and CPV has been found in the CNS of pups with systemic parvovirus infections, in the absence of CNS manifestations in some cases. However, the presence of mild inflammatory changes in the CNS of the affected pups is not characteristic of the pure hereditary forms of leukoencephalopathy.
Neurologic Diseases of Suspected Infectious Origin and Prion Disease
Granulomatous Meningoencephalitis
Etiology and Pathogenesis
Clinical Findings

Diagnosis
Clinical Laboratory Findings
Medical Imaging Findings
Surgical Biopsy
Molecular Genetic Testing
Pathologic Findings
Therapy
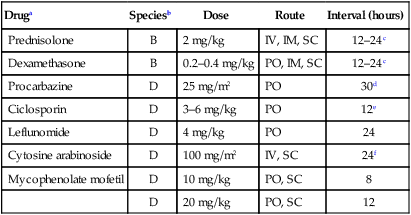
Druga
Speciesb
Dose
Route
Interval (hours)
Prednisolone
B
2 mg/kg
IV, IM, SC
12–24c
Dexamethasone
B
0.2–0.4 mg/kg
PO, IM, SC
12–24c
Procarbazine
D
25 mg/m2
PO
30d
Ciclosporin
D
3–6 mg/kg
PO
12e
Leflunomide
D
4 mg/kg
PO
24
Cytosine arabinoside
D
100 mg/m2
IV, SC
24f
Mycophenolate mofetil
D
10 mg/kg
PO, SC
8
D
20 mg/kg
PO, SC
12
Necrotizing Encephalitis
Etiology and Pathogenesis
Clinical Findings
Diagnosis
Pathologic Findings
Therapy
Hydrocephalus with Periventricular Encephalitis in Dogs
Etiology
Diagnosis
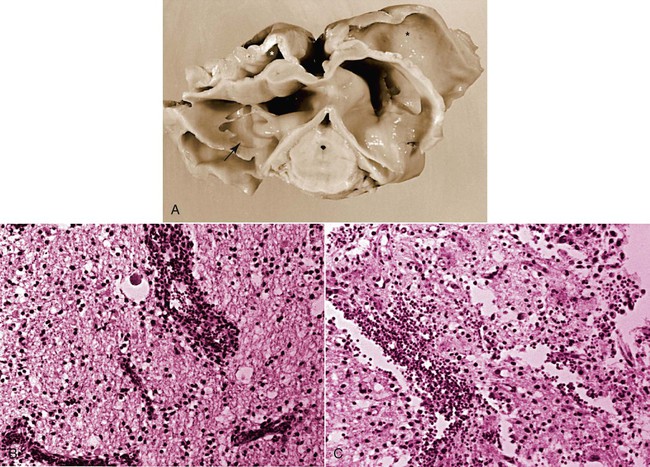
Pathologic Findings
Therapy
Nonsuppurative Meningoencephalitis in Greyhounds
Canine Leukoencephalopathy and Parvovirus Infection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree