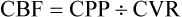Neuroanesthesia for South American Camelids
Why Might South American Camelids Require Brain Neuroanesthesia?
SACs may suffer from a variety of neurologic diseases, including congenital defects such as hydrocephalus, in which the neonatal nature of the animal also has to be taken into consideration.1 Infectious and parasitic diseases such as listeriosis, equine herpes virus, West Nile Virus, rabies, meningeal worm, and brain abscesses and meningoencephalitis from a multitude of pathogens may all cause neurological signs in the SAC.2–9 Careful handling and disinfection of anesthesia equipment are needed in these cases to prevent transmission of pathogens to other patients or staff. Neoplasia has also been reported as a cause of space-occupying lesions causing neurologic signs in llamas and alpacas.10,11
Neuroanesthesia for the Brain
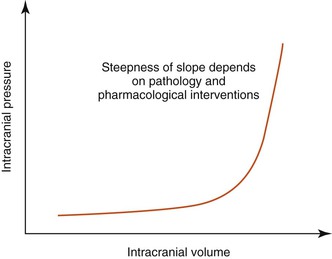
The Monroe-Kellie Doctrine
The skull is a rigid, bony box. In most mammals, the intracranial space contains the following:
If the volume of one component increases, it has to be balanced by a decrease in another. However, the volume of brain parenchyma cannot be altered easily, although intracranial blood volume and intracranial CSF volume may be altered by a small amount. So, for example, if brain parenchyma volume increases (e.g., through a space-occupying lesion such as an abscess), then to compensate, some of the CSF may move into the subarachnoid space around the spinal cord. However, at a certain point the compensatory mechanisms are exhausted, and intracranial pressure rises, often producing clinical signs (Figure 51-1 and Box 51-1).
Intracranial Pressure Regulation
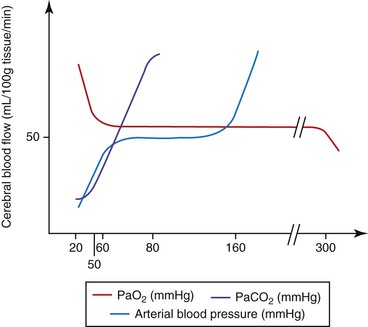
Brain parenchyma may increase in volume because of edema, hemorrhage, inflammation, or space-occupying lesions. CSF volume may increase from an increased production (e.g., choroid plexus tumor) or decreased drainage (e.g., caused by space-occupying lesions). The volume of blood in the intracranial contents may be increased for a variety of reasons. Increased central venous pressure (CVP) may be caused by the head lying lower than the heart, occluding the jugular veins, Valsalva maneuvers (forced expiration against a closed glottis), and intermittent positive pressure ventilation (IPPV) with high inspiratory pressures. Intracranial blood volume is also affected by the degree of vasodilation or vasoconstriction, that is, vasomotor tone. Cerebral vasomotor tone is normally “responsive” to a number of stimuli (Figure 51-2 and Table 51-1). As seen in Table 51-1, within the “normal” range for arterial blood pressure, ICP should not be altered by small changes in blood pressure. This is because the cerebral circulation is usually governed by “autoregulation”; that is, cerebral blood flow (CBF) is maintained over a wide range of blood pressures. It must be remembered, however, that in a “diseased brain” autoregulation may not function “normally.”
TABLE 51-1
Cerebral Vascular Response to Changes in Arterial Carbon Dioxide, Oxygen, and Mean Blood Pressure
| Vasodilation | Vasoconstriction | |
| PaCO2 | With increasing partial pressures, up to 80 mm Hg | With decreasing partial pressures, down to 20 mm Hg |
| PaO2 | <50 mm Hg | >300 mm Hg |
| Mean arterial blood pressure | >160 mm Hg | <60 mm Hg |
PaCO2, Partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen.
Why Is Elevated Intracranial Pressure a Problem?
Cerebral perfusion pressure (CPP) = Mean arterial pressure (MAP) − Intracranial pressure (ICP)
Thus we can see that if MAP is maintained and ICP rises, then CPP will be reduced.
Both CPP and cerebrovascular resistance (CVR) affect CBF, according to the following formula:
This formula underscores the importance of vasomotor tone in influencing CBF.
How Can We Alleviate Raised Intracranial Pressure?
Furosemide (1–2 mg/kg) is also a potent diuretic and is often used in conjunction with mannitol. If given before mannitol, furosemide may protect against transient increase in ICP, as mentioned above. Besides acting as a diuretic, furosemide also reduces the production of CSF. The use of glucocorticoids is controversial, and even if advocated, the doses, individual compounds, and dosing regimens are as yet not agreed upon. Potential beneficial effects (at least in non-trauma cases) include diuresis, membrane stabilization, and reduction of inflammation edema.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree