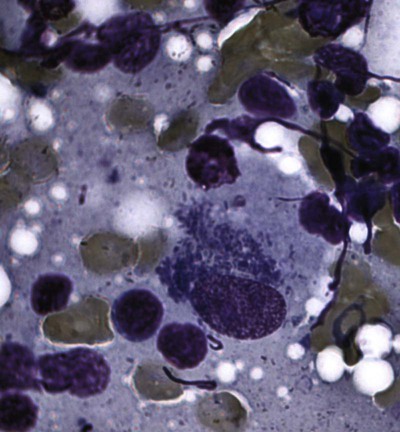
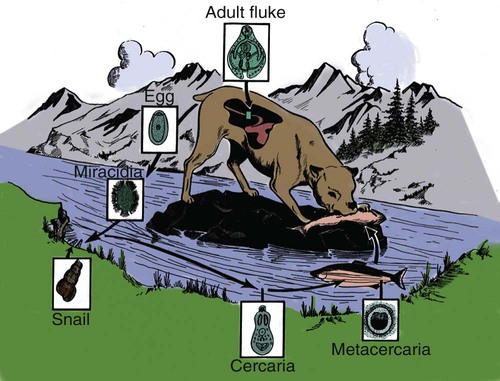
Shimon Harrus, Trevor Waner, and T. Mark Neer Neorickettsia (previously Ehrlichia) risticii is the agent of equine monocytotropic neorickettsiosis or Potomac horse fever. N. risticii was reclassified because of its close relationship with Neorickettsia helminthoeca, the etiologic agent of salmon poisoning disease (see later discussion) and Neorickettsia (previously Ehrlichia) sennetsu, the agent of human monocytotropic neorickettsiosis (Sennetsu fever). These agents are transmitted by invertebrate intermediate hosts, rather than tick vectors. See Table 25-1 for a summary of these organisms relative to their vectors, reservoir hosts, and geographic distribution. The detection of N. risticii DNA in aquatic insects has raised the possibility that these arthropods may serve as a vectors of infection.4 N. risticii in horses has been determined to be acquired experimentally through ingestion of infected snails.55 The vector of N. sennetsu has not been determined, but as with N. helminthoeca, transmission may involve the ingestion of raw fish containing the pathogenic organisms that were acquired by the fish ingesting an infected snail. TABLE 25-1 Monocytotropic Neorickettsia Species Infecting Domestic Animals and Humans Entero, Enterocytes; macro, macrophages; mast, mast cells; mono, monocytes; ?, uncertain. aNaturally infected hosts have also been experimentally infected; however, they are not also listed in this column. Some of these experimental infections are subclinical or transient. Both domestic and wild canids are naturally susceptible to N. helminthoeca infection. bTrematode infected snails; Pleuroceridae (Elimia livescens, Juga yrekaensis). Although cats can be experimentally infected with N. risticii, the significance of natural infection in cats has yet to be determined. There is some evidence to suggest that N. risticii may result in clinical disease in naturally infected cats.22 Experimental inoculation of six cats with N. risticii has confirmed that cats are susceptible to infection.17 In this study, N. risticii was only isolated from blood of two cats that developed clinical signs of disease. Clinical signs were limited to acute depression, mild fever and anorexia, intermittent diarrhea, and lymphadenomegaly. All cats inoculated with N. risticii seroconverted between 10 and 23 days posinoculation. There were no clearly defined hematologic changes associated with infection. Natural infection with N. risticii has been recognized in a number of cats.2,8,8 Attempts to isolate the organism from five naturally infected cats were not successful; however, Western blot analysis of sera from the cats revealed antibodies to four of the nine major antigens of N. risticii.64 A serosurvey of cats (n = 344) in the United States established that 14% of cats tested had serum antibody reactive to N. risticii alone, and a further 5% had those reacting with both N. risticii and Ehrlichia canis.53,85 No gender or age predilection was detected. However, a relationship between N. risticii antibody increase and outdoor cats or cats displaying signs of vomiting was found. A serosurvey of cats in Spain revealed that 3 of 122 cats (2.4%) had antibodies reactive with N. risticii.2 Serum samples of 2 of these cats were also reactive with E. canis. All the blood samples analyzed by polymerase chain reaction (PCR) yielded negative results. The authors concluded that until isolation, PCR, and sequencing can be performed, the results should be interpreted with caution, because this infectious agent has been seldom reported outside North America. The mode of natural infection of N. risticii in cats is unknown but probably involves an arthropod vector. In one study, however, that checked for the presence of N. risticii in cat fleas (Ctenocephalides felis) taken from cats residing in Alabama, Maryland, and Texas, all fleas had negative test results for the presence of N. risticii by PCR.53 The detection of N. risticii DNA in aquatic insects has raised the possibility that these arthropods may serve as a route of infection.4 N. risticii has been acquired experimentally in horses through ingestion of infected snails.55 The likelihood that N. risticii could be transmitted via blood transfusions should be considered because serologic studies have demonstrated that exposure to N. risticii does exist in cats in the natural setting. Therefore, some authors suggest screening cats for the presence of antibodies to N. risticii before their use as blood donors.71 For further information on the role of blood transfusions in transmission of this and other infections, see Chapter 93. Limited studies have been conducted to define the pathogenesis and the course of the acute disease. There is, however, clinical evidence that N. risticii organisms may persist after treatment.64 The sequence of events in five naturally infected cats indicated that despite standard treatment protocols, there was still persistence of N. risticii organisms and this continued to result in hematologic abnormalities in these cats. No clear laboratory abnormalities were demonstrated in cats after experimental infection with N. risticii.1,17 However, abnormal findings have been described in five naturally infected cats, including anemia, leukopenia, lymphopenia, thrombocytopenia, and dysproteinemia.64 Oral doxycycline (5 mg/kg, every 12 hours for 21 days) has been effectively used to treat cats with feline neorickettsiosis. However, this regime was not fully successful in treating five naturally infected (seropositive) cats that had recurring thrombocytopenia and leukopenia until the dose per kilogram was doubled.64 The cats were treated over a period of 200 days, and at the end of the treatment the authors concluded that repeated or prolonged periods of antibacterial treatment may be indicated if clinical and hematologic response is not observed despite the elimination of detectable antibodies. A number of species of the genus Neorickettsia are documented to infect dogs. See Table 25-1 for a summary of these organisms relative to their vectors, reservoir hosts, and geographic distribution. Some serologic data indicate that N. risticii may be widely distributed in dogs throughout the United States, although one serosurvey in North Carolina and Virginia indicated no evidence of exposure.86 Conversely, 38% of dogs from Thailand (n = 49) with signs of fever, anemia, or thrombocytopenia were found to have antibodies reactive with N. risticii.87 Serologic reactivity to N. risticii has also been demonstrated in dogs from northwestern Spain,3 but the possibility of serologic cross-reaction with other ehrlichial organisms has to be considered in these latter studies. Dogs experimentally infected with N. risticii showed no clinical signs.75 In another report, six dogs with suspected naturally acquired infection had a wide range of signs that included fever, bleeding tendencies, edema, neurologic signs, polyarthritis, anemia, and thrombocytopenia. In these six dogs, it was not certain whether the etiologic agent was truly N. risticii or a canine-adapted strain of the equine pathogen or possibly another rickettsial organism. The dogs had low positive indirect fluorescent antibody titers for N. risticii, and the results were confirmed by Western immunoblotting. In these dogs, N. risticii was isolated and propagated from blood samples and the agent was visualized by electron microscopic examination. Identification of the agent was then confirmed by PCR, targeting the partial 16S rRNA gene (100% sequence similarity in 719 base pairs). The authors termed the condition seen in these dogs as “atypical canine ehrlichiosis.” Overall, tetracycline therapy has been found to be an effective treatment in dogs proposed to have been naturally infected with N. risticii. However, in one dog the organism persisted in the blood long after treatment had been initiated.44 John R. Gorham, William J. Foreyt, and Jane E. Sykes Salmon poisoning disease (SPD), or salmon disease, is a helminth-transmitted rickettsial disease of domestic and wild Canidae that occurs on the western slopes of the Cascade Mountains from northern California to central Washington (Fig. 25-1). The disease is often fatal if untreated. Occasionally, cases of SPD occur outside the indigenous range of the disease in areas where infected fish migrate or are transported. The presence of cases in British Columbia may indicate that the indigenous range of the disease is greater than previously reported.6 An SPD-like disease, which is similar phylogenetically and antigenically, has been reported in dogs from Brazil.38,40 The etiologic agent of SPD is Neorickettsia helminthoeca, a coccoid or coccobacillary rickettsia that is approximately 0.3 µm in size.16,65 Pleomorphic rods up to 2 µm long, sometimes bent in rings or crescents, have been observed. The gram-negative rickettsial organisms appear purple with Giemsa stain, red with Macchiavello’s stain, black or dark brown with Levaditi’s method, and pale blue with hematoxylin and eosin stain. The rickettsiae almost fill the cytoplasm of cells of the mononuclear phagocyte system (MPS) that they primarily infect (Fig. 25-2). The rickettsiae have been grown in canine monocytes,29 canine leukocytes and sarcoma cells, mouse lymphoblasts, and a macrophage cell line.61,74 Antigenically and genetically, N. helminthoeca is closely related to Ehrlichia spp. Based on 16S rRNA gene sequences, N. helminthoeca is most closely related to N. risticii, the agent of Potomac horse fever, and N. sennetsu, the agent of human sennetsu fever in Japan, Malaysia, and other areas of Asia (see Table 25-1).20 In dead fish, rickettsiae in metacercariae (encysted trematode larvae) of Nanophyetus salmincola do not survive 30 days at 4° C.28 In lymph nodes, organisms resist freezing at −20° C for 31 to 158 days66; they remain viable in leukocytes at 4.5° C and 52.5° C for 48 hours and 2 minutes, respectively, but not at 60° C for 5 minutes.83 At −80° C the agent can be maintained in cell culture fluid for up to 3 months.12 It is highly likely that the Elokomin fluke fever (EFF) agent23 is another strain of N. helminthoeca.29,30 The disease in dogs associated with the EFF agent results in high morbidity but a lower mortality than SPD. It appears that metacercariae can harbor EFF and SPD agents simultaneously. EFF is rarely recognized as a distinct entity in naturally occurring disease. Histologically, EFF infections in dogs are similar to but less severe than those seen with SPD. In a survey of 331 practitioners in endemic areas, 35% reported that they had diagnosed SPD in dogs that had been treated previously for SPD.32 Although it has been generally accepted that dogs surviving SPD infection had a solid immunity, the data now suggest that other strains such as EFF may be pathogenic under field conditions,6 or the initial SPD infection failed to evoke a durable immunity.7 The vector of SPD is a trematode, N. salmincola, which harbors the rickettsiae throughout its life cycle stages from egg to adult.47 Three different hosts are required for the completion of the trematode life cycle: snails, fish, and mammals or birds (Fig. 25-3). Lists of intermediate and definitive hosts of the fluke can be found elsewhere.47 The snail intermediate host, Oxytrema silicula, is a pleurocerid that inhabits fresh or brackish stream water in coastal areas of Washington, Oregon, and northern California. Areas of trematode infection, therefore, depend on the distribution of O. silicula. Cercariae (free-swimming trematode larvae) leave the snail and penetrate the second intermediate host, which is usually a salmonid fish, certain species of nonsalmonid fish, or the Pacific giant salamander (Dicamptodon ensatus). The metacercariae usually localize in the kidneys of fish (Fig. 25-4) but can be found in any tissue and in slime on the skin of the fish. Fish are infected in freshwater and retain the trematode and the rickettsial infection throughout their ocean migration before returning to freshwater up to 3 years later.92 Adult trematodes develop in the intestine approximately 6 days after the ingestion of metacercariae-infected fish, fish skin, or entrails by dogs and certain other fish-eating mammals that serve as definitive hosts, such as bears and raccoons, and certain birds. Clinical signs of rickettsial disease occur in Canidae, primarily dogs, foxes, and coyotes. However, two captive polar bears receiving long-term glucocorticoid therapy for skin conditions succumbed to an SPD-like disease after eating inadequately frozen salmon,79 and an SPD-like disease was also reported in two captive Malayan sun bears after they were fed live trout from a northern California reservoir.31 Cats are not susceptible to SPD, but trematodes develop when infected fish are ingested.47 SPD also has been transmitted by parenteral injection of infected blood, spleen and lymph suspensions, adult flukes, helminth-infected snail livers, and helminth eggs. Partial transmission success was obtained by allowing ticks (Haemaphysalis leachi and Rhipicephalus sanguineus) that had fed on infected dogs to subsequently feed on susceptible dogs and by parenteral injection of suspensions of R. sanguineus into dogs.65 Susceptible dogs also have been experimentally infected with cell-cultured Neorickettsia organisms74 and aerosolized lymph node suspensions from infected dogs; on rare occasions, direct transmission of infection between dogs has been suspected.7 After ingestion of raw or partially cooked, metacercariae-infected salmonid fish by a susceptible dog, the fluke matures, and the adult stage attaches to the mucosa of the intestine and by some unknown mechanism inoculates the rickettsiae (Fig. 25-5). Initial replication of rickettsiae probably takes place in the epithelial cells of the villi or the intestinal lymphoid tissue. Inflammation of the solitary lymphoid follicles and Peyer’s patches along the intestinal tract contributes to enteritis. Mild enteritis may be observed in dogs infected only with the flukes but without rickettsiae. Rickettsiae enter the blood early in the course of the disease and spread to the lymph nodes, spleen, tonsils, thymus, liver, lungs, and brain.30 Although secondary bacterial infections often occur, the exact cause of death in SPD is unknown. Sepsis may result from translocation of bacteria across the damaged intestinal wall. Severe hypoalbuminemia and anemia secondary to gastrointestinal (GI) hemorrhage and fluid losses may contribute. Investigations to demonstrate a toxin have been limited. The signs of SPD are consistent in all Canidae. The usual incubation period after the ingestion of parasitized fish is 5 to 7 days, although some dogs have incubation periods as long as 19 to 33 days. The first sign usually is a sudden febrile response, which typically reaches a peak of 40° C to 42° C (104° F to 107.6° F; Fig. 25-6). The temperature gradually decreases to normal or below normal over the next 4 to 8 days. Dogs are frequently hypothermic when death occurs 7 to 10 days after the initial clinical evidence of infection. Some animals show only a slight increase in temperature or a shortened febrile period; however, they may still die if left untreated. Inappetence or complete anorexia always accompanies or follows the onset of fever. Affected animals often continue to have inappetence throughout the course of the disease. Affected dogs are usually depressed. Marked weight loss and weakness may follow. Within 14 days of eating infected fish, coyotes on a controlled experiment lost approximately 58% of their body weight when compared with uninfected coyotes.28 Diarrhea and vomiting may occur, with the diarrhea becoming progressively worse and often consisting primarily of blood at the time of death. Affected animals occasionally exhibit extreme thirst and drink copious quantities of water. A serous nasal discharge may develop early in the febrile period. Neurologic signs have been described in some affected dogs.88 Later, a mucopurulent conjunctival exudate may be seen. Enlarged mandibular, superficial cervical, and popliteal lymph nodes can be palpated as early as 5 days after infection. SPD-infected dogs may show severe GI signs, which are often clinically indistinguishable from canine parvoviral enteritis (see Chapter 8). Distemper represents another possible differential diagnosis. Hematologic and biochemical findings of SPD-infected domestic dogs are often nonspecific, and total leukocyte counts have ranged from leukopenia to leukocytosis.74 Laboratory results of 45 dogs representing 17 breeds and various mixed breeds that were naturally infected with SPD are listed in Web Table 25-1.54 Thrombocytopenia was present in 88% of the dogs tested. Lymphopenia (77%), eosinophilia (77%), increased serum alkaline phosphatase activities (64%), and reduced serum albumin concentrations (49%) were other frequent findings.40 In experimentally infected coyotes, significantly higher numbers of band cells; lower numbers of eosinophils; lower serum concentrations of creatinine, glucose, calcium, and inorganic phosphorus; and lower albumin and alkaline phosphatase activities were detected when compared with uninfected coyotes.28 WEB TABLE 25-1 Results of Initial Laboratory Tests in Dogs with Salmon Poisoning
Neorickettsia and Wolbachia Infections
Neorickettsia Risticii Infection
Species (Diseases)
Geographic Distribution
Infected Cell Type
Vector
Infected Hosts
Reservoir
Natural, Domestica
Experimental
Neorickettsia risticii (equine monocytotropic ehrlichiosis)
United States, Canada
Mono, entero
Trematode
Snailsb: Elimia livescens, Juga yrekaensis
Aquatic insects?
Horses
Dogs, cats, mice, nonhuman primates
N. risticii (subsp. atypicalis)
United States
Mono, mast, entero
?
?
Dogs
ND
Neorickettsia helminthoeca (salmon poisoning disease, Elokomin fluke fever)
Northwest coastal United States
(Brazil ?; see text)
Mono, macro
Trematodes: Nanophyetus salmincola
First: Snail Oxytrema silicula
Second: Fish (salmonids) and Pacific giant salamander
Dogs
Bears
Dogs (by inoculation of infected tissues or fluids of trematodes or hosts, or by ticks; see text)
Neorickettsia sennetsu (human Sennetsu fever)
Japan, Malaysia, other areas in Asia
Mono
Trematode
First: Snail?
Second: Fish
Human
ND

Cats
Etiology and Epidemiology
Pathogenesis
Diagnosis
Clinical Laboratory Findings
Therapy
Dogs
Neorickettsia Helminthoeca Infection (Salmon Poisoning Disease)
Etiology
Salmon Disease Agent
Elokomin Fluke Fever Agent
Epidemiology
Pathogenesis
Clinical Findings
Salmon Poisoning Disease
Diagnosis
Clinical Laboratory Findings and Radiographic Imaging
Normal Range
Maximum
Minimum
Mean
SD
Number of Dogs above NR
Number of Dogs below NR
Number of Dogs Tested
Dog age (years)
12
0.3
5
3.7
NA
NA
45
WHOLE BLOOD VALUES
Leukocytes (per µL)
6000–17,000
66,000
4200
12,800
10,600
6
6
44
Mature neutrophils
3000–11,500
62,604
1792
10,908
10,296
13
1
44
Band neutrophils
0–300
2548
0
168
430
8
0
44
Lymphocytes
1000–4800
3776
92
791
667
0
34
44
Eosinophils
100–1250
891
0
77
202
0
34
44
Monocytes
150–1350
4137
0
824
811
10
0
44
Hematocrit (%)
37–55
54
25
43
7
1
8
43
Platelet counts (×103/dL)
200–500
377
16
113
108
0
14
16
Fibrinogen (mg/dL)
200–400
700
100
400
160
14
3
38
SERUM BIOCHEMICAL VALUES
Sodium (mEq/L)
145–154
159
126
141
8
1
13
16
Potassium (mEq/L)
4.1–5.3
5.3
3.4
4.4
0.5
0
3
16
Chloride (mEq/L)
105–116
134
84
106
12
1
4
14
Total CO2
16–26
24
15
18
3
0
4
10
Calcium (mg/dL)
9.9–11.4
10.8
7.3
9.1
0.9
0
30
37
Phosphorus (mg/dL)
3.0–6.2
7.9
3.0
4.8
1.5
7
0
36
Creatinine (mg/dL)
0.8–1.6
1.7
0.5
1.1
0.5
2
2
17
Urea nitrogen (mg/dL)
8–31
90
9
22
19
6
0
37
Glucose (mg/dL)
70–118
133
44
92
17
4
2
37
ALT (IU/L)
19–102
499
21
92
83
9
0
37
AST (IU/L)
15–66
274
24
90
60
17
0
35
Alkaline phosphatase (IU/L)
15–150
2098
60
254
339
23
0
36
Total protein (g/dL)
5.4–7.4
8.2
3.9
5.9
1.1
4
11
37
Albumin (g/dL)
2.5–3.5
3.3
1.6
2.5
0.4
0
18
37
Total bilirubin (mg/dL)
0–0.4
2.0
0
0.4
0.6
4
0
37
Globulins (mg/dL)
2.9–3.9
5.8
1.9
3.4
0.9
9
10
37
Cholesterol (mg/dL)
135–300
408
99
215
85
8
6
37 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neorickettsia and Wolbachia Infections