CHAPTER 6 With the recognition that tumor development is a stepwise process, potentially preneoplastic changes have assumed new diagnostic and clinical significance. These changes include hyperplasia (increased cell number in a tissue), metaplasia (transformation of one differentiated cell type into another), and dysplasia (abnormal pattern of tissue growth) (Fig. 6-1). Hyperplasia, which is an increase in the number of cells in a tissue, should be distinguished from hypertrophy, which is an increase in individual cell size rather than number. Metaplasia is seen most commonly in epithelial tissue. In several species of animals, vitamin A deficiency is characterized by squamous metaplasia of respiratory and digestive epithelium. Dysplasia usually refers to disorderly arrangement of cells within epithelium. In general, preneoplastic changes are reversible. They arise in response to physiologic demands, injury, or irritation and resolve with the removal of the inciting factor. For example, epidermal hyperplasia is a normal part of wound repair, and skeletal muscle hypertrophy is an adaptive response to increased workload. Preneoplastic changes often indicate an increased risk for neoplasia in the affected tissue, and preneoplastic lesions may progress to neoplasia. The terms dysplasia and metaplasia can be applied to tumors to describe changes that persist during the transition from preneoplasia to neoplasia; however, the terms hyperplasia and hypertrophy are not appropriate in descriptions of true neoplasms. Fig. 6-1 Preneoplastic changes that may precede tumor emergence. Mesenchymal tumors arise in cells of embryonic mesodermal origin. Benign tumors originating from mesenchymal cells are usually named by adding the suffix -oma to the name of the cell of origin. Thus a lipoma is a benign tumor derived from a lipocyte (“fat cell”) (Fig. 6-2, A), and a fibroma is a benign tumor of fibroblast origin. A malignant tumor of mesenchymal origin is a sarcoma (“fleshy growth”). A prefix or modifier indicates the tissue of origin. For example, a liposarcoma is a malignant tumor of lipocyte origin (Fig. 6-2, B), and a fibrosarcoma is a tumor composed of malignant fibroblasts. The cells of the hematopoietic system are mesenchymal. Tumors arising from circulating blood cells or their precursors are termed leukemias (“white blood”); neoplastic hematopoietic cells are usually found in large numbers in the bloodstream (Fig. 6-3), although they may also form solid tumor masses. Fig. 6-2 Comparison of benign and malignant tumors of lipocyte origin, dog. Fig. 6-3 Acute lymphoid leukemia, peripheral blood, dog. Benign tumors that arise from glandular epithelium are called adenomas, regardless of their microscopic appearance. However, the term is also applied to many tumors that are derived from nonglandular epithelial tissues but that have a tubular appearance such as renal adenomas. The term papilloma refers to a benign exophytic growth arising from an epithelial surface, whereas a polyp is a grossly visible, benign epithelial tumor projecting from a mucosal surface (Fig. 6-4). Fig. 6-4 Polyp, small intestine, mouse. Mixed tumors contain multiple cell types derived from a single or multiple germ layers. Mixed tumors are believed to arise from a single pluripotent or totipotent cell capable of differentiating into a variety of more mature cell types. Teratomas and teratocarcinomas arise from totipotential germ cells; thus they contain tissue derived from all embryonic cell layers and consist of a bizarre mixture of adult and embryonic tissue types. The mixed mammary gland tumor of dogs is generally considered a mixed tumor. A mixed mammary tumor is composed of a variable admixture of neoplastic epithelial elements (luminal epithelium and myoepithelium) and mesenchymal elements (fibrous connective tissue, fat, cartilage, and bone) (Fig. 6-5). In Web Table 6-1, the names of common benign neoplasms in animals and their malignant counterparts are shown. The names given are those commonly employed in veterinary medicine. The terms used by veterinary pathologists to describe tumors in animals may differ from the terms used by medical pathologists to describe human tumors. This is partly because conventional usage plays an important role in tumor nomenclature; thus tumor nomenclature may be dictated by historic precedent rather than by logic. Moreover, attempts to standardize diagnostic terms for tumors in veterinary medicine have lagged far behind such efforts in the medical arena. A significant difference between veterinary and human nomenclature is that a benign tumor arising from melanocytes is termed a benign melanoma or melanocytoma by veterinary pathologists and a nevus by medical pathologists. Medical pathologists reserve the term melanoma for a malignant tumor of melanocyte origin, whereas veterinary pathologists term such tumors malignant melanomas. Benign Versus Malignant Tumors The most important distinction between benign and malignant tumors is that malignant tumors are able to invade locally and metastasize systemically, but benign tumors are not. The invasive capabilities of malignant tumors are associated with enhanced tumor cell motility, increased production of proteases, and altered tumor cell adhesion characteristics. Although benign tumors are ultimately distinguished from their malignant counterparts based on invasiveness, a variety of morphologic and behavioral features are generally considered to predict the potential for malignant behavior (Table 6-1). Although both benign and malignant tumors are composed of proliferating cells, malignant tumors have essentially unlimited replicative potential. The tumors are relatively independent of exogenous growth stimulatory molecules and are insensitive to growth inhibitory signals from their environment. Moreover, malignant cells are better able than benign cells to evade programmed cell death (apoptosis) and to escape the host’s cytotoxic immune response. Compared with benign tumors, malignant tumors stimulate marked angiogenesis (the formation of new blood vessels), thus assuring adequate tumor nutrition. Morphology: Each normal, fully differentiated, mature tissue type has a characteristic gross and microscopic appearance that varies little from individual to individual of a species. Neoplastic tissues lose these differentiated features of cellular morphology and organization to a variable extent. In general, malignant tumors appear less differentiated than benign tumors. Loss of morphologic hallmarks of tissue maturity is often accompanied by loss of functional capacity and development of aggressive behavior. Neoplastic cells often show considerable morphologic variability compared with the normal tissue from which they are derived. Tumor cells, especially malignant tumor cells, may exhibit anaplasia (cellular atypia). Anaplastic cells are poorly differentiated cells that exhibit notable cellular and nuclear pleomorphism (variation in size and shape). In some tumors, bizarre tumor giant cells are seen (Fig. 6-6). Nuclei may exhibit extreme variability in number, size, shape, chromatin distribution, and nucleolar size and number (Fig. 6-7). Anaplastic nuclei are often hyperchromatic (darkly staining) because of increased DNA content; are disproportionately large relative to cell size, resulting in an increased nuclear : cytoplasmic ratio; and have prominent nucleoli. Mitotic figures in tumor cells may be numerous. Many of the nuclear changes seen in neoplastic cells reflect the frequent cell division, chromosomal abnormalities, and active metabolic state that characterize these cells. Fig. 6-6 Anaplastic liposarcoma, subcutis, dog. Fig. 6-7 Anaplastic bronchioalveolar carcinoma, dog. Neoplastic cells often exhibit loss of characteristic cytoplasmic and nuclear features. For example, poorly differentiated mast cell tumors often lack the prominent cytoplasmic granules that are a hallmark of normal mast cells (Fig. 6-8). Special stains or immunohistochemistry may be able to highlight some characteristic morphologic feature retained in at least a subpopulation of tumor cells. As an example, characteristic granules may be revealed in some cells of feline and canine mast cell tumors by staining with toluidine blue or Giemsa. Many tumor cells have noticeably basophilic cytoplasm as a result of the presence of large numbers of ribosomes required for rapid cell growth and frequent cell division. Fig. 6-8 Mast cell tumor, skin, cat. In tumors, normal tissue organization is usually lost to some extent. Increasing loss of normal architecture in tumors correlates with increasing independence of tumor cells from their surrounding tissue. As an example, lymphomas arising in lymph nodes often consist of solid sheets of neoplastic cells that partially or completely efface normal lymph node architecture (Fig. 6-9). In tissue that normally undergoes continual renewal, such as the skin and oral mucosa, the normal maturation sequence may be altered. Thus in squamous cell carcinomas, the orderly morphologic progression from basal cell layer to fully keratinized stratum corneum may not be seen (Fig. 6-10). Fig. 6-9 Lymphoma (lymphosarcoma), lymph node, dog. Fig. 6-10 Squamous cell carcinoma, tongue, cat. Function: Loss of differentiated function frequently accompanies loss of differentiated morphology in tumors. Thus neoplastic cells arising from alveolar lining cells of the lung generally fail to perform normal respiratory functions and tumors of primitive germ cell origin do not form normal sperm or ova. Some aspects of normal function may be retained. Thyroid adenomas may continue to produce thyroid hormones, and plasma cell tumors may secrete immunoglobulins. However, in the majority of cases, these functions are no longer regulated appropriately because the neoplastic cells have lost responsiveness to and dependence on normal regulatory pathways. Thus thyroid adenomas may produce clinical hyperthyroidism, and plasma cell tumors may cause hypergammaglobulinemia. Behavior: Benign tumors are generally expansile and may compress adjacent tissue, whereas malignant tumors have invasive and in many instances metastatic capabilities. In malignant tumors, alterations in adhesion, motility, and protease production allow tumor cells to leave the tumor mass and penetrate surrounding tissue. Moreover, for malignant cells to invade and ultimately metastasize, they must become completely independent of local growth regulatory controls and acquire an independent blood supply. Acquisition of these features allows tumors to spread well beyond their ordinary anatomic niches. Totipotent stem cells, such as embryonic stem cells, can give rise to all tissues of the body, whereas multipotent or pluripotent stem cells can give rise to a smaller variety of tissue types. The plasticity of most adult stem cells is generally considered to be relatively restricted. Leukemias provide excellent examples of neoplasms arising from stem cells. A leukemia almost always arises from a single hematopoietic stem cell that has undergone heritable genetic change. The progeny of this stem cell all exhibit the same genetic change, although the cell type and degree of differentiation of the progeny may vary. Thus in myelogenous leukemia, a neoplastic multipotential stem cell may give rise to a combination of leukemic cells of the granulocytic, monocytic, and erythroid series (Fig. 6-11). The concept of a stem cell origin for cancer explains not only the embryonic characteristics of neoplastic cells but also the success of treatment strategies that use differentiating agents such as retinoids (vitamin A derivatives used to induce maturation of some human leukemia cells). Fig. 6-11 Myelomonocytic leukemia, peripheral blood, dog. The cell cycle consists of G1 (presynthetic), S (DNA synthesis), G2 (premitotic), and M (mitotic) phases (Fig. 6-12). Quiescent cells are in a physiologic state called G0. In adult tissue, many cells reside in G0 and are unable to enter the cell cycle at all or do so only when stimulated by extrinsic factors. Moreover, in response to DNA damage, even actively dividing normal cells undergo cell-cycle arrest, usually at one of several cell-cycle checkpoints. Cell-cycle arrest is initiated by the multifunctional tumor suppressor gene product p53 and gives the cell time to repair DNA damage. Fig. 6-12 Cell-cycle landmarks. Continuously Dividing Tissues (Labile Tissues): In continuously dividing tissues (also called labile tissues), cells proliferate throughout life, replacing those that are lost. These tissues include surface epithelia, such as stratified squamous surfaces of the skin, oral cavity, vagina, and cervix; the lining mucosa of all the excretory ducts of the glands of the body (e.g., salivary glands, pancreas, and biliary tract); the columnar epithelium of the gastrointestinal tract and uterus; the transitional epithelium of the urinary tract; and cells of the bone marrow and hematopoietic tissue. In most of these tissues, mature cells are derived from stem cells, which have an unlimited capacity to proliferate and whose progeny may differentiate into a variety of mature cell types. Quiescent Tissues (Stable Tissues): Quiescent (or stable) tissues normally have a low level of replication; however, cells from these tissues can undergo rapid division in response to stimuli and are thus capable of reconstituting the tissue of origin. They are considered to be in the G0 stage of the cell cycle but can be stimulated to enter G1. This category includes the parenchymal cells of the liver, kidneys, and pancreas; mesenchymal cells, such as fibroblasts and smooth muscle; vascular endothelial cells; and resting lymphocytes and other leukocytes. The regenerative capacity of stable cells is best exemplified by the ability of the liver to regenerate after partial hepatectomy and after acute chemical injury. Fibroblasts, endothelial cells, smooth muscle cells, chondrocytes, and osteocytes are quiescent in adult mammals but proliferate in response to injury. Fibroblasts in particular may proliferate extensively. Nondividing Tissues (Permanent Tissues): Nondividing (permanent) tissues contain cells that have left the cell cycle and cannot undergo mitotic division in postnatal life. Neurons and skeletal and cardiac muscle cells belong to this group. If neurons in the central nervous system (CNS) are destroyed, the tissue is generally replaced by the proliferation of the CNS supportive elements, the glial cells. However, recent results demonstrate that limited neurogenesis from stem cells may occur in adult brains. Although mature skeletal muscle cells do not divide, skeletal muscle does have some regenerative capacity, through the differentiation of the satellite cells that are attached to the endomysial sheaths. If the ends of severed muscle fibers are closely juxtaposed, muscle regeneration in mammals can be excellent, but this is a condition that can rarely be attained under practical conditions. Cardiac muscle has very limited, if any, regenerative capacity, and extensive injury to the heart muscle, as may occur in myocardial infarction, is followed by scar formation. In adult tissues, the size of a cell population is determined by the relative rates of cell proliferation, differentiation, and death. Fig. 6-13 depicts these relationships and shows that increased cell numbers may result from either increased proliferation or decreased cell death. Fig. 6-13 Mechanisms regulating cell populations. Proliferation: Cell proliferation is largely controlled by signals (soluble or contact-dependent) from the microenvironment that either stimulate or inhibit cell proliferation. An excess of stimulators or a deficiency of inhibitors leads to net growth. Although accelerated growth can be accomplished by shortening the cell cycle, the most important mechanism of growth is the conversion of resting or quiescent cells into proliferating cells by making the cells enter the cell cycle. Both the recruitment of quiescent cells into the cycle and cell-cycle progression require stimulatory signals to overcome normal physiologic blocks to cell proliferation. Cell proliferation can be stimulated under both physiologic and pathologic conditions. The proliferation of mammary epithelium under hormonal stimulation during lactation is an example of physiologic proliferation. Pathologic conditions, such as tissue injury, cell death, and mechanical alterations, also stimulate cell proliferation. Excessive physiologic stimulation may create pathologic conditions, such as enlargement of the thyroid, as a consequence of increased serum levels of thyroid-stimulating hormone. Differentiation: Differentiation also impacts the size of a cell population and its proliferative potential. For example, myocytes and neurons are terminally differentiated cells (i.e., they are at an end stage of differentiation and are not capable of replicating). In some adult tissues, such as liver and kidney, differentiated cells are normally quiescent but are able to proliferate when necessary. In proliferative tissue, such as bone marrow and the epithelia of the skin and gut, the mature cells are terminally differentiated, short-lived, and incapable of replication, but they may be replaced by new cells arising from stem cells. Thus in such tissues there is a homeostatic equilibrium between the proliferation of stem cells, their differentiation, and the death of fully differentiated cells. Cell Death: A variety of cell death mechanisms, including senescence, apoptosis, and autophagy, eliminate irreversibly damaged or effete cells to maintain normal tissue homeostasis. In response to DNA damage, oxidative stress, and telomere shortening, proliferating cells may undergo a permanent arrest in the G1 phase of the cell cycle termed cellular senescence. Senescence is mediated by activation of the p53 or retinoblastoma pathways of cell cycle arrest. Senescent cells often express senescence-associated β-galactosidase. Apoptosis is a form of “programmed cell death” that serves both as a normal physiologic process and as a response to pathologic stimuli. In proliferative tissue, such as gut epithelium, terminally differentiated cells undergo apoptosis and are thus removed from the cell population. Apoptosis may occur in response to withdrawal of survival factors from the cell environment or by binding of death factors, such as Fas ligand and tumor necrosis factor-α (TNF-α) to cell surface receptors. Hypoxia and lack of essential nutrients may end in apoptosis. DNA damage may also induce apoptosis; in this case, apoptosis is triggered by p53. Apoptosis may be stimulated by the activity of cytotoxic immune cells, including T lymphocytes and natural killer (NK) cells. Signals for apoptosis activate a variety of signaling pathways, many of which ultimately result in the release of cytochrome C from mitochondria. The final effectors of apoptosis are the caspases, intracellular proteases that selectively destroy cellular organelles and degrade genomic DNA into nucleosome-sized fragments. The morphologic hallmarks of apoptosis include margination of chromatin, condensation and fragmentation of the nucleus, and condensation of the cell with preservation of organelles. Ultimately, the cell breaks into membrane-bound apoptotic bodies that are engulfed by surrounding cells without stimulating an inflammatory response (Fig. 6-14). Fig. 6-14 Lymphoma, apoptosis, lymph node, horse. Essentially unlimited proliferative potential is a hallmark of neoplasia, especially of malignant neoplasms. Unlike normal cells, many tumor cells are immortal. This immortality is due to a combination of the alterations discussed later. In general, neoplastic cells escape normal limits on cell division, become independent of external growth stimulatory and inhibitory factors, and lose their susceptibility to apoptotic signals. This results in an imbalance between cell production and cell loss and a net increase in tumor size. However, it should be noted that the growth of a tumor is not completely exponential. A proportion of tumor cells is continually lost from the replicative pool because of irreversible cell-cycle arrest, differentiation, and death (Fig. 6-15). Fig. 6-15 Schematic representation of tumor growth. Latency: As illustrated in Fig. 6-16, the latent period for a tumor is the time before a tumor becomes clinically detectable. The smallest clinically detectable mass is about 1 cm in diameter and contains about 109 cells. To form a tumor that size, a single transformed cell must undergo about 30 rounds of cell division, if all the progeny remain viable and capable of replication. Thus, by the time most tumors become clinically evident, they have probably been developing in the host for many years. However, once tumors reach a clinically detectable size, their growth may appear to be very rapid, because only 10 doubling cycles are required to convert a 1-g tumor into a 1-kg tumor. In fact, volume doubling times for tumors vary considerably, depending on the rate at which tumor cells divide, the fraction of tumor cells that are replicatively competent, and the rate at which tumor cells die. In general, benign neoplasms grow more slowly than malignant tumors, although there is considerable variation among tumors. Moreover, tumors may grow erratically, depending on their blood supply, the effect of extrinsic growth-regulating factors such as hormones, the efficacy of the host immune response, and the emergence of subpopulations of particularly aggressive tumor cells. Fig. 6-16 Biology of solid tumor growth. Proliferation: Many neoplastic cells no longer respond to extrinsic or intrinsic signals directing them into G0 and no longer express functional p53. Thus the cells move continuously through the cell cycle. Moreover, because the tumor cells do not undergo cell-cycle arrest after DNA damage, they progressively accumulate potentially mutagenic DNA damage (Fig. 6-17). For homeostasis to be maintained, normal cells must engage in a continual dialogue with their environment. There is a constant exchange of information among cells via soluble mediators, including growth stimulatory factors, growth inhibitory factors, and hormones. These soluble mediators tightly control the growth of nonneoplastic cells. Neoplastic cells, on the other hand, often lose both their dependence on extrinsic growth stimulatory substances and their susceptibility to growth inhibitory signals from their environment. The mechanisms by which this occurs are discussed later. The end result is that tumor cells are no longer responsive to the needs of the organism as a whole and develop the capacity to drive their own replication. Fig. 6-17 Schematic representation of the cell cycle. The mitotic index is usually defined as the number of tumor cells in a microscopic field that contain condensed chromosomes and lack nuclear membranes (Fig. 6-18). Such cells are interpreted as being actively dividing, and the mitotic index of a tumor is considered to indicate its malignant potential. However, the mitotic index can be misleading. The fraction of tumor cells observed to be in mitosis depends not only on the number of cells undergoing mitosis but also on the length of time required to complete the process. In tumor cells, the time required for completion of the cell cycle is generally as long as or even longer than for normal cells. Mitotic figures may persist in cells unable to complete cell division and abnormal mitotic figures may be seen. Differentiation: As discussed previously, tumor cells are generally less differentiated than normal cells. In some instances, however, some tumor cells can be forced to differentiate into more mature, near-normal cells. Leukemia cells are particularly susceptible to differentiation therapy, and retinoids are routinely employed to treat acute promyelocytic leukemia in human patients. Other differentiating agents, including vitamin D compounds and cytokines, have been less effective in this disease. Vitamin D compounds are showing some promise in differentiation therapy of human epithelial tumors, and compounds that epigenetically alter tumor cells by modifying the histones in chromatin may also enhance differentiation of tumor cells (discussed later). A common assumption underlying differentiation therapies is that more differentiated tumor cells will have a less stem cell-like phenotype and will thus have reduced proliferative potential. Cell Death: Because the DNA replication machinery is unable to duplicate the extreme ends of DNA templates, the telomeres that form the ends of chromosomes are shortened at each cell division. Embryonic cells express telomerase, a riboprotein enzyme that allows telomeres to be replicated and even expanded; however, most adult cells do not express this protein and their telomeres shrink with each round of cell division. Very short telomeres are incompatible with continued cell division and trigger cellular senescence in normal cells. However, many neoplastic cells regain the ability to produce telomerase and thus to replicate their telomeres. Reexpression of telomerase appears to play an important role in the escape of tumor cells from senescence and their consequent immortality. Neoplasms develop as the result of multiple genetic and epigenetic changes that occur over a relatively long time course. It is the cumulative effect of these alterations that creates a tumor. The stepwise evolution of tumors has been studied most thoroughly in carcinomas. There are several types of carcinoma that develop in an orderly and predictable fashion. For instance, squamous cell carcinoma arises from the epithelium of the eyelid in many species of animals, including cattle, horses, cats, and dogs. In all species, these tumors develop through the same sequence of steps: epidermal hyperplasia, carcinoma in situ, and invasive carcinoma. Extensive studies of experimentally induced squamous cell carcinomas in the skin of mice have revealed a similar morphologic pattern of tumor evolution (Fig. 6-19) and have led to a detailed model of stepwise carcinoma development as described in the next section (Fig. 6-20). Fig. 6-19 Development of squamous cell carcinoma in the skin of a hairless mouse exposed to ultraviolet (UV) radiation. Fig. 6-20 Illustration of stepwise tumor development. Most tumors are believed to be of clonal origin (i.e., they are ultimately derived from a single transformed cell). Tumor cell heterogeneity is generated during the course of tumor growth by the progressive accumulation of heritable changes in tumor cells (see Fig. 6-16). With each new genetic alteration, the progeny of the genetically altered tumor cell constitute a subclone of tumor cells. The generation of subclones is fostered by the marked genetic instability of tumor cells compared with normal cells. Successful subclones are those that have a high proliferative rate, are able to evade the host immune response, can stimulate the development of an independent blood supply, are independent of exogenous growth factors, and are able to escape from the primary tumor and spread to distant sites. These characteristics give successful subclones a selective advantage over other subclones of cells within the tumor. A tumor subclone with a selective advantage will eventually predominate. When cancers arise on the surface of an abdominal or thoracic structure, they encounter few anatomic barriers to spread. Thus mesotheliomas may be confined to the abdominal or pleural cavities, but the tumor cells within these cavities readily spread to cover all visceral and parietal surfaces (Fig. 6-21). In both humans and dogs, ovarian adenocarcinomas preferentially spread transcoelomically. Although such tumors are rare in dogs, they are commonly encountered in women. Even in the absence of invasion into the underlying organs, tumors such as mesotheliomas and ovarian adenocarcinomas are extremely difficult to treat and are generally fatal. Fig. 6-21 Mesothelioma, peritoneum of the abdominal cavity, dog. In general, most carcinomas metastasize via the lymphatic system, although sarcomas may also employ this route of spread. The pattern of lymph node involvement is usually dictated by preexisting routes of regional lymphatic drainage. The lymph nodes closest to the tumor are usually colonized earliest and develop the largest metastatic tumor masses (Fig. 6-22). Thus adenocarcinomas of the intestine in all species usually metastasize first to the mesenteric lymph nodes and later to other lymph nodes within and outside the abdominal cavity. For many years, it was assumed that cancers spread in a stepwise manner from the primary site to regional lymph nodes, then to distant sites, such as the lung, and that regional lymph nodes actually represented a mechanical barrier to the spread of cancer. Thus removal of regional lymph nodes containing tumor tissue was believed to protect the patient from further spread of the tumor. However, regional lymph nodes may be bypassed as a result of natural, tumor-related, or treatment-induced anomalies in lymphatic drainage. More recent studies suggest that lymphatic spread does not occur in an orderly fashion and that metastasis to regional lymph nodes indicates that neoplastic disease has become widely systemic. Fig. 6-22 Pancreatic carcinoma, metastatic, hepatic (portal) lymph node, dog. Because lymphatic vessels connect with the vascular system, the distinction between lymphatic and hematogenous spread is somewhat artificial. However, sarcomas do tend to use the hematogenous route of spread more frequently than carcinomas. Tumors generally invade veins rather than arteries because arterial walls are much thicker and more difficult to penetrate. Tumors that enter veins ultimately enter the vena cava and lodge in the lungs (Fig. 6-23) or enter the portal system and lodge in the liver. Neoplasms metastatic to the lungs may secondarily enter the arterial circulation. Some tumors have a notable predilection for veins. Pheochromocytomas of many species frequently enter the adrenal veins, where they may form large tumor masses extending into the vena cava.
Neoplasia and Tumor Biology*
Definitions
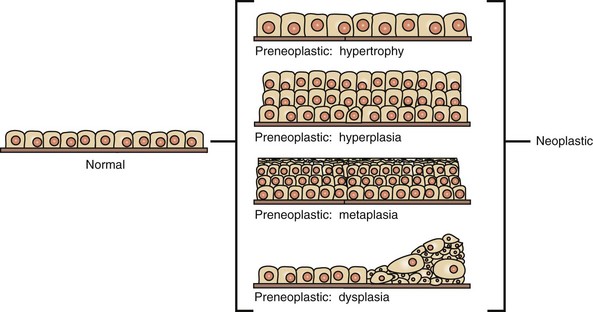
Preneoplastic changes in tissues include alterations in cell number, size, and organization. In this example, preneoplastic changes are illustrated in simple cuboidal epithelium, although such changes may also occur in other epithelial and mesenchymal cell types. (Redrawn with permission from Dr. D.F. Kusewitt, College of Veterinary Medicine, The Ohio State University.)
Nomenclature
Mesenchymal Tumors
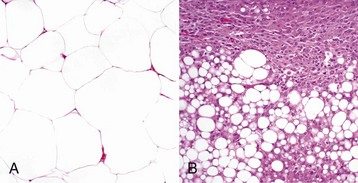
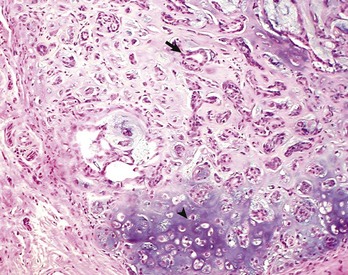
A, The benign lipoma is composed of mature fat cells indistinguishable from normal cells. H&E stain. B, The liposarcoma consists of poorly differentiated cells, many of which do not have the morphologic features characteristic of lipocytes. H&E stain. (Courtesy College of Veterinary Medicine, The Ohio State University.)
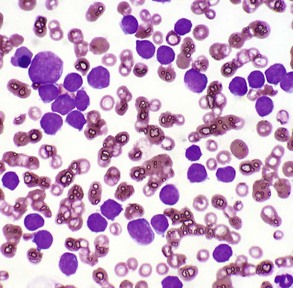
The peripheral blood smear contains numerous neoplastic large lymphocytes. Flow cytometry identified these cells as B lymphocytes. The white blood cell count of this animal was 293,000 leukocytes/µL. Wright’s stain. (Courtesy Dr. M.L. Wellman, College of Veterinary Medicine, The Ohio State University.)
Epithelial Tumors
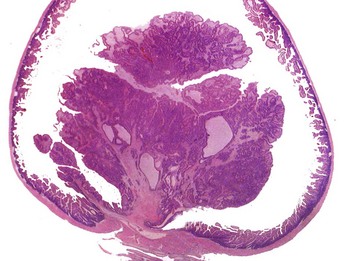
The neoplastic growth arises from the mucosa and extends into the lumen of the intestine. There is no invasion of the intestinal wall. H&E stain. (Courtesy College of Veterinary Medicine, The Ohio State University.)
Mixed Tumors
Veterinary Nomenclature
Tumor Characteristics
Differentiation
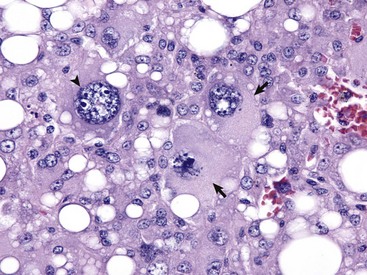
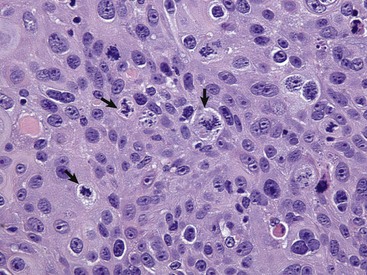
Anaplastic tumors of epithelial or mesenchymal cell origin often contain bizarre tumor giant cells such as the cells indicated by the arrows. Also, note the large nuclei with abundant coarsely aggregated chromatin and multiple nucleoli (arrowhead). H&E stain. (Courtesy College of Veterinary Medicine, University of Illinois.)
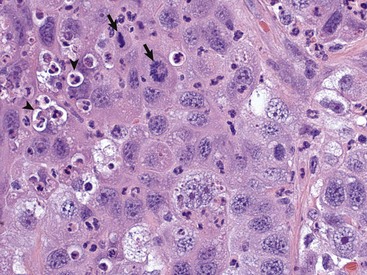
This tumor exhibits marked nuclear pleomorphism and has a fairly high mitotic index. Note the prominent mitotic figures (arrows) and phagocytosis of neutrophils by the tumor cells (emperipolesis) (arrowheads). H&E stain. (Courtesy Dr. J. F. Zachary, College of Veterinary Medicine, University of Illinois.)
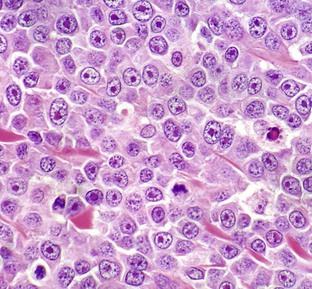
With H&E staining, hallmark mast cell granules are not visible. To see these granules, the section must be stained with a metachromatic stain such as toluidine blue or Giemsa. Note the very large single nucleolus and marginated chromatin in the neoplastic cells. H&E stain. (Courtesy College of Veterinary Medicine, The Ohio State University.)
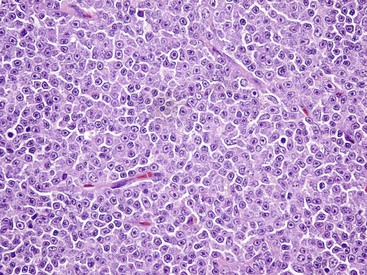
The normal lymph node architecture has been completely effaced by solid sheets of neoplastic lymphocytes that are relatively uniform in morphology. H&E stain. (Courtesy College of Veterinary Medicine, The Ohio State University.)
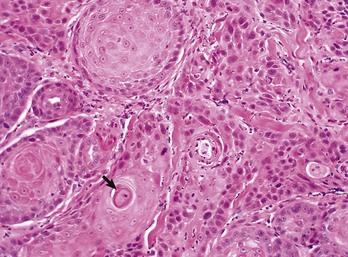
The orderly pattern of epidermal maturation seen in normal oral mucosa is absent from this squamous cell carcinoma. An occasional “keratin pearl” (arrow) reveals the tissue of origin for this tumor. H&E stain. (Courtesy College of Veterinary Medicine, The Ohio State University.)
Stem Cells and Differentiation
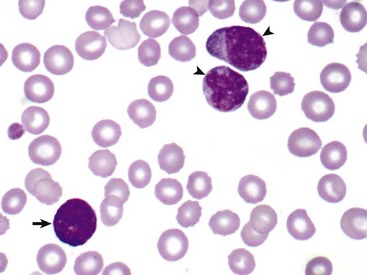
In this unusual case, leukemic cells of both monocytic (arrowheads) and granulocytic (basophil) (arrow) origin were present in peripheral blood. The animal had a marked leukocytosis (103,000 white blood cells/µL) and thrombocytopenia. Wright’s stain. (Courtesy Dr. M.J. Burkhard, College of Veterinary Medicine, The Ohio State University.)
Proliferation
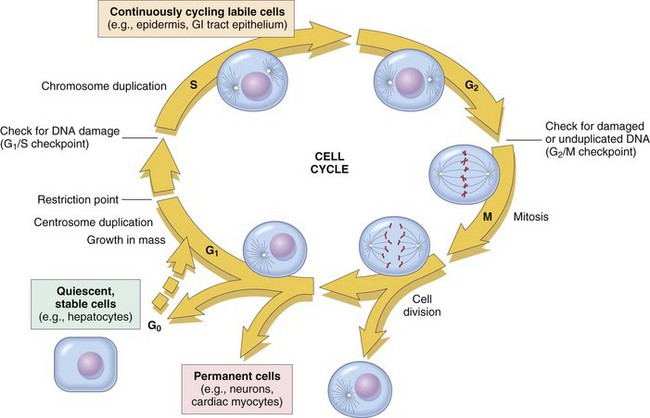
The figure shows the cell-cycle phases (G0, G1, G2, S, and M), the location of the G1 restriction point, and the G1/S and G2/M cell-cycle checkpoints. Cells from labile tissues, such as the epidermis and the gastrointestinal (GI) tract, may cycle continuously; stable cells, such as hepatocytes, are quiescent but can enter the cell cycle; permanent cells, such as neurons and cardiac myocytes, have lost the capacity to proliferate. (Modified from Pollard TD, Earnshaw WC: Cell biology, Philadelphia, 2002, Saunders.)
Proliferative Activity in Nonneoplastic Tissue
Normal Tissue Growth
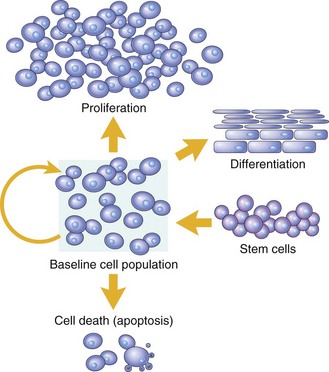
Cell numbers can be altered by increased or decreased rates of stem cell input, by cell death due to apoptosis, or by changes in the rates of proliferation or differentiation. (Modified from McCarthy NJ, Smith CA, Williams GT: Cancer Metastasis Rev 11:157-178, 1992.)
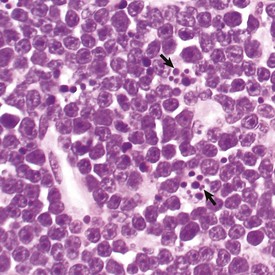
The light microscopic appearance of apoptosis is characterized by condensation and fragmentation of nuclei (arrows), cell shrinkage, engulfment of apoptotic bodies by surrounding cells, and lack of inflammation. H&E stain. (Courtesy Dr. R. Tan, College of Veterinary Medicine, University of Illinois.)
Tumor Growth
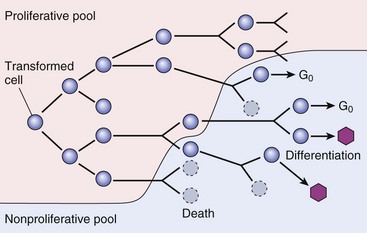
As the cell population expands, a progressively higher percentage of tumor cells leave the replicative pool by reversion to G0, differentiation, and death. (From Kumar V, Abbas A, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
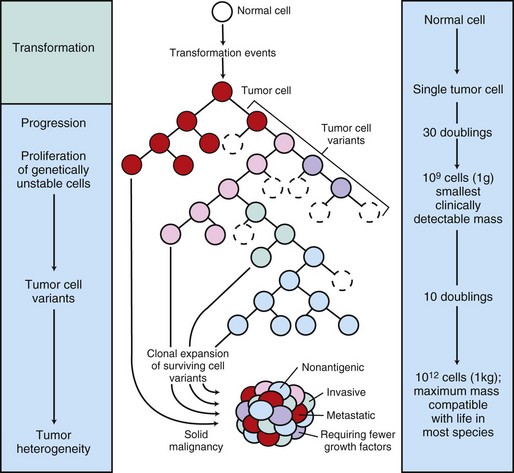
The center panel illustrates clonal evolution of a tumor and generation of tumor-cell heterogeneity. New subclones arise from descendents of the original transformed cell. With progressive growth, the tumor mass becomes enriched for those variants that are more adept at evading host defenses and are likely to be more aggressive. The left panel shows the corresponding stages of tumor progression, and the right panel depicts minimal estimates of tumor-cell doublings that precede the formation of a clinically detectable tumor mass. It is evident that by the time a solid tumor is detected, it has already completed a major portion of its life cycle, as measured by population doublings. The maximum tumor size compatible with life depends to some extent on the species affected. (Modified from Kumar V, Abbas A, Fausto N: Robbins & Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders.)
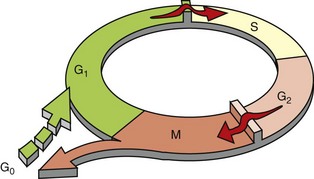
Many normal cells reside in G0, a nonreplicative state. When they do enter the proliferative cycle, they can arrest at cell-cycle checkpoints at the G1-S and G2-M boundaries in response to a variety of stimuli, including DNA damage. In contrast, tumor cells spend little time in G0 and often do not undergo cell-cycle arrest in response to DNA damage or lack of extrinsic growth stimuli. (Modified from Kumar V, Abbas A, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders.)
Tumor Evolution
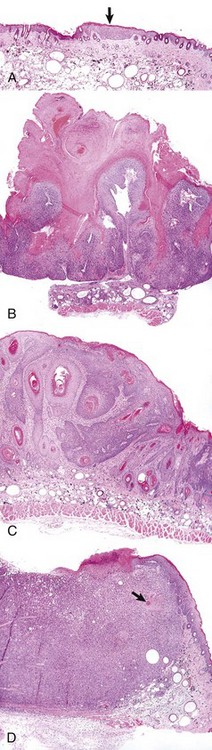
A, A focus of epidermal hyperplasia (arrow) is the earliest lesion seen. B, This develops into a papilloma, a benign exophytic papillary growth that is highly keratinized and does not penetrate into the dermis. C, As the papilloma undergoes conversion into a malignant squamous cell carcinoma, it begins to invade the dermis and to lose the regular pattern of epithelial differentiation. D, A fully developed squamous cell carcinoma has lost most differentiated characteristics and extends deep into the dermis. Only a few keratin “pearls” (arrow) indicate the origin of this tumor from the epidermis of the skin. All figures were taken at the same magnification. H&E stain. (Courtesy Dr. T.M. Oberyszyn, The Ohio State University.)
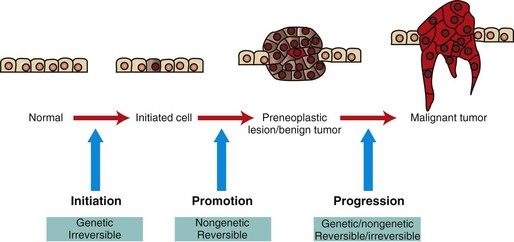
Initiated cells have irreversible genetic damage. In the presence of a promoter, these initiated cells expand to form a preneoplastic lesion or benign tumor. With further genetic and epigenetic alterations, a malignant tumor emerges from a subclone of cells within the benign precursor lesion. (Redrawn with permission from Dr. D.F. Kusewitt, College of Veterinary Medicine, The Ohio State University.)
Tumor Heterogeneity and Clonal Selection
Tumor Spread
Pathways of Tumor Metastasis
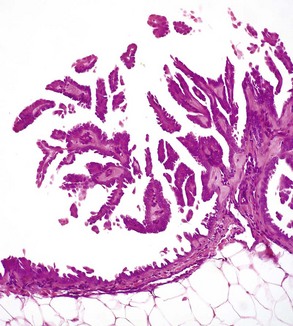
Mesotheliomas spread extensively within body cavities, but rarely metastasize by lymphatic or hematogenous routes. Note in the figure that neoplastic mesothelial cells cover the serosal surfaces and form papillary fronds, but they do not infiltrate underlying tissue. H&E stain. (Courtesy College of Veterinary Medicine, The Ohio State University.)
Lymphatic
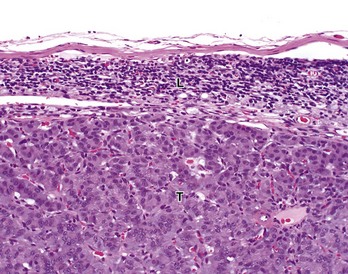
Tumor cells (T) have almost completely replaced the normal architecture of the lymph node, except for a thin subcapsular rim of lymphocytes (L). The tumor was confirmed by immunohistochemistry to be a functional pancreatic islet β-cell carcinoma. H&E stain. (Courtesy College of Veterinary Medicine, The Ohio State University.)
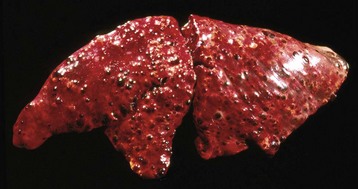
Hematogenous
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neoplasia and Tumor Biology