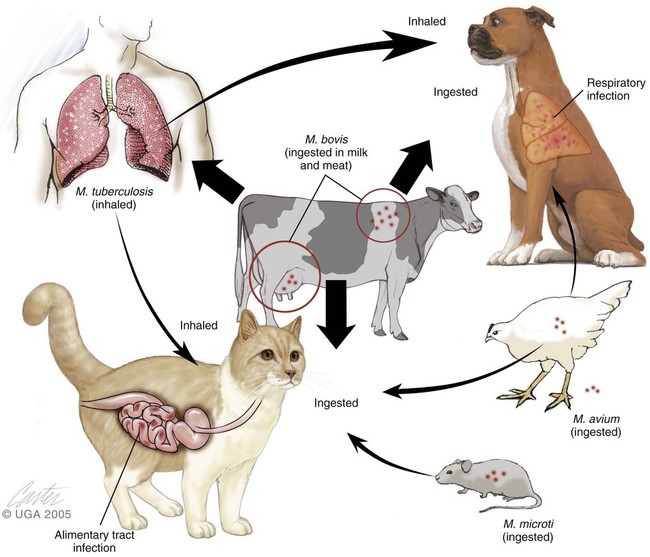
Mycobacterial infections are caused by bacteria that belong to the family Mycobacteriaceae, order Actinomycetales. Mycobacterium is a genus comprising morphologically similar, aerobic, non-spore-forming, nonmotile, pleomorphic bacterial rods with wide variations in host affinity and pathogenic potential. Historically, these bacteria have been subdivided into several groups and individual species (Table 48-1). Genetic sequencing studies have corroborated and extended this taxonomic classification. Molecular diagnostics have become more useful than phenotypic traits by classifying isolated mycobacteria according to their genetic relationships. Although cultural characteristics are less useful, growth rates and pigment formation continue to be a practical means of classifying these bacteria in the laboratory, and biochemical characteristics often correlate with the virulence and form of disease produced in the mammalian host. TABLE 48-1 Characteristics of Selected Species of Mycobacterium of Veterinary Interest FI, Facultative intracellular; OI, obligate intracellular; S, saprophyte; ?, uncertain. aIncluded in tuberculous group in this chapter because produces clinically similar disease. Growth enhanced by glycerol and 42°C. bBecause of taxonomic uncertainties, organisms of M. chelonae and M. abscessus are hyphenated. Craig E. Greene and Danielle A. Gunn-Moore There are other members of the M. tuberculosis complex. Mycobacterium canettii is a human pathogen, and Mycobacterium africanum is a rare cause of human tuberculosis in Africa. Mycobacterium microti is a predominantly rodent pathogen that infects voles, but it can also infect a much wider host range, including cats, people, many wild animals, South American camelids, and even a dog.* M. microti was previously, rather confusingly, termed M. microti–like because it had properties intermediate between those of M. tuberculosis and M. bovis, and it was not clear at the time that it actually was M. microti. Another synonym for M. microti was “M. tuberculosis–M. bovis variant,” a label applied when it was identified as a common cause of tuberculous infection in cats in Great Britain.31,71,123,124 Mycobacterium pinnipedii is an organism that infects seals. Other opportunistic, saprophytic mycobacteria that occur as granuloma-producing pathogens, which sometimes disseminate, are organisms related to and including M. avium. Results of genetic analysis have resulted in a proposal to separate M. avium strains into M. avium subsp. avium for those animal isolates coming from birds and M. avium subsp hominissuis for those from swine or human origin.73a,127,223a Considerable overlap occurs between the properties of M. avium strains and a closely related pathogen Mycobacterium intracellulare. Because of this indistinct separation, M. avium–M. intracellulare, or M. avium complex (MAC) has been used to refer to these organisms. In other classification systems, they are labeled nontuberculous mycobacteria or mycobacteria other than tuberculosis-complex organisms, and they produce granulomas but not true tubercles. Their slow growth during cultivation makes them similar to tuberculous bacteria. In adult humans, MAC organisms produce pulmonary infiltrates and disseminated disease, generally in immunocompromised hosts. In cats and children, localized lymphadenitis can occur. When newer methods of genetic analysis have been performed, some cases clinically classified as feline leprosy syndrome (see next section) have been attributed to MAC infection. MAC disease often becomes disseminated in cats and dogs. As an opportunistic disease, MAC infection is more likely in immunocompromised hosts.118 Among opportunistic mycobacteria in people and animals, MAC organisms are the most likely to produce bacteremia and multiple-organ disseminated disease. Serotyping by agglutination reactions has been classically used to differentiate MAC isolates. Presently, nucleic acid probes are used for their rapid identification. Using serologic methods, MAC organisms consist of 28 serovars; 1 through 6 and 8 through 11 are assigned to M. avium, and 7, 12 through 17, 19, 20, and 25 are assigned to M. intracellulare.152,304 Serotypes 1 and 4 have been isolated from cats, and serotypes 1, 2, and 4 have been isolated from dogs.162 Currently, MAC organisms are distinguished by nucleic acid profiles (see Organism Detection later in this chapter). Humans are the only reservoir hosts for M. tuberculosis. Dogs and cats are susceptible to infections with M. tuberculosis and M. bovis. Cats are naturally much more resistant to M. tuberculosis than they are to M. bovis. Canine and feline infections with M. tuberculosis are considered an anthropozoonosis—that is, the direction of transmission is from people to animal (Fig. 48-1). Although pets acquire the infection from people, spread back from dogs or cats to people has not been reported. Dogs have had a higher prevalence of infection with M. tuberculosis than have cats. Dogs with tuberculous pneumonitis discharge organisms in the sputum as do infected people. Aerosolized droplets are the primary means of transmission of this disease. Airborne droplet nuclei from respiratory secretions fall to the ground where they temporarily remain viable but stationary and thus relatively noninfectious for other people and pets. Only small (3 to 5 µm) diameter particles can successfully bypass upper respiratory clearance mechanisms and deposit in alveoli. Discharges that are not airborne may potentially be infectious to dogs and cats exposed through close contact. In general, tubercle bacilli are not as transmittable as other bacterial pathogens because prolonged, frequent exposure or large inocula are usually required. Because of measures imposed to control infection in people, the overall prevalence of human and animal M. tuberculosis infections had been decreasing in developed countries. Relative increases have occurred in densely populated urban areas and in economically depressed areas. The interrelated factors of homelessness, illicit drug use, and human immunodeficiency virus (HIV) infection have caused unanticipated increases in its prevalence. Multidrug-resistant tuberculosis has emerged in these affected populations because of irregular compliance with drug therapy. Pets in such environments will have an increased risk of becoming infected. The incidence of M. tuberculosis infections in the United States is highest in Atlantic coast metropolitan regions and in the southeastern regions. M. tuberculosis infection has become an important emerging anthropozoonosis in free-ranging wildlife. Initially the infection was identified only in wildlife or captive zoo animals such as elephants that had close and prolonged contact with people.219,232 Infections caused by M. tuberculosis have been increasing, especially in underdeveloped countries, as a result of the acquired immunodeficiency syndrome (AIDS) epidemic. In Africa, expansion of ecotourism and changing land use have increased contact between infected people and free-living animals. Wild animals living in close proximity to human habitations and refuse sites have become infected. Certain wildlife populations have become endemically infected, such as the banded mongoose (Mungos mungo) in Botswana and suricates (Suricata suricatta) in South Africa.8 This organism has a wide host range, including many animals and people, and a worldwide geographic distribution.126 In most industrialized countries, bovine tuberculosis has been controlled with surveillance, testing and slaughter, and pasteurization of dairy products. In developing countries, tuberculosis caused by M. bovis has become widespread and a potential risk for exposed people and animals.63 With respect to M. bovis infections, the gastrointestinal (GI) tract is the most common portal of entry. Cats and dogs can be potential disseminators of disease when the organism preferentially localizes in the intestinal or respiratory tracts. Because of localization of infection, cats usually excrete the organism via feces and dogs via sputum. M. bovis does not persist long in the environment, and reservoir hosts are essential for survival of the organism. Outside its hosts, the organism survives for a period varying from 4 days in summer to less than 28 days in winter.155 However, organisms may persist for several months in organic material such as feces or carcasses.273,297,297 Dogs and cats acquire infection when they consume contaminated milk or meat. Dogs and cats may be involved in the maintenance of bovine tuberculosis on farms where it is enzootic and may be rarely responsible for transmission of the bovine bacillus to people (see Fig. 48-1).326 Subclinically, infected dogs and cats sometimes remain on farms after reactor cattle have been identified and removed from the herd, and farms or families with recurrent tuberculous infections should have their pet animal contacts checked periodically. Rarely, spread of M. bovis between people has been reported. Cats are more commonly infected with the bovine bacillus than dogs are, and on an experimental basis cats appear to be more susceptible to bovine than to human tubercle bacilli. Part of this affinity is related to the frequent ingestion of contaminated, unpasteurized milk or uncooked meat or offal from infected cattle, or from eating M. bovis–infected wild rodents. Milk is an ideal medium for the organism because it buffers the gastric acid that normally prevents colonization of the lower GI tract with tubercle bacilli. As a result of eradication measures, the prevalence of bovine tuberculosis is low in the United States. In Argentina, domestic cats are often fed raw bovine lung, which may be an important source of their infection with M. bovis.338 Increased use of commercial pet foods and trends to urban living have reduced the prevalence of bovine tuberculosis in dogs and cats. Despite the reduction of infection in domestic animals, bovine tuberculosis remains a problem worldwide, except in Australia, because it has become established in wildlife hosts that have acquired infection from cattle, and wildlife act as continued reservoirs for infection. As a result, domestic animals such as cattle, cats, and dogs continue to become infected.78,79,164,259,314 For example, in the United States, white-tailed deer (Odocoileus virginianus) in Michigan are infected and provide a reservoir of infection for cattle and other domestic animals including the dog and cat.164,326 In Great Britain and Ireland, badgers (Meles meles) have become a major wildlife reservoir.75 Although transmission between badgers is predominantly by aerosols, M. bovis has also been found in their bite wounds, suggesting that cutaneous inoculation occurs. After an outbreak in cattle from Cornwall, United Kingdom, infection in domestic cats on the farm and local badgers all shared the same spoligotype.229 Interestingly, the fact that no cattle had been recently kept on the premises suggested that local badgers had somehow infected the resident cat population. Because cats and badgers are unlikely to exhibit the intimate interaction necessary for cross-infection, cats are most likely to be infected by eating secondarily infected small wild mammals, which are commonly found in endemic areas.75,77 In New Zealand, feral brushtail possums (Trichosurus vulpecula) act as reservoir hosts; wild ferrets, pigs, cats, introduced deer species, and goats serve as amplifier hosts when their carcasses infect carrion-eating animals. Amplifier hosts can become infected from carnivorous ingestion. Sheep, horses, and hedgehogs are spillover hosts that are incidentally infected.61 Stoats, dogs, hares, rabbits, and many small rodents are occasional dead-end hosts. Captive and free-living exotic wildlife carnivores, including nondomestic canids and felids, and domestic and feral dogs and cats have been most commonly infected with M. bovis. Ingestion of infected carrion is the most common source of infection. The infection is maintained in gregarious herbivores, while carnivorous predators such as lions (Panthera leo), cheetahs (Acinonyx jubatus), leopards (Panthera pardus), spotted hyenas (Crocuta crocuta), Iberian lynx (Lynx pardina), European lynx (Lynx lynx), Siberian tigers (Panthera tigris longipilis), snow leopards (Uncia uncia), and feral domestic cats have become secondarily infected.* Additional subsequent transmission of infection via aerosol or biting between gregarious carnivores, such as lions, has been suspected.169 Infection with M. microti has been seen most frequently in Great Britain, predominantly in rural cats with avid hunting behavior. The source of infection is believed to be prey species.31,122,124,148 The disease is a spillover from infection of wildlife. Some previous reports of this infection may have been mistaken for M. bovis infections.124 However, the areas where cats become infected with M. microti and M. bovis are geographically distinct.284 Furthermore the genotypes of M. microti are geographically clustered within the endemic areas. M. microti infection has also been documented in a dog with peritonitis.73 Infection from rodents and South American camelids is considered the source of infection for cats and people. Pulmonary infection was found in the people who had no underlying evidence of immunodeficiency. These organisms are ubiquitous worldwide in soil and water under certain conditions. MAC organisms have been shown to be present in acidic (pH 5.0 to 5.5) conditions and soils high in organic matter.175 These conditions are met in acidic swamp areas, coastal plains, and brackish coastal waters. Feces of infected birds contain large numbers of bacilli, and infection of dogs and cats occurs from ingestion of infected meat or contact with infected soil or with fomites contaminated by poultry carcasses or feces (see Fig. 48-1). Unlike M. tuberculosis and M. bovis, MAC organisms remain viable for at least 2 years in the environment, including municipal water supplies, soil, dairy products, and tissues of birds and mammals. Natural, potable, and even treated water supplies in temperate and tropical regions can harbor nontuberculous mycobacteria. These organisms can exist within biofilms that coat the internal surfaces of water pipes, fixtures, and storage tanks.190 MAC organisms are likely to be found in recirculating water systems commonly used in human hospitals.252 Hot water has a higher degree of colonization, and chlorine does not inactivate these organisms, especially at higher temperatures. In hospital environments, bathing, rinsing, lavage, and disinfectant solutions made with potable water can become contaminated with MAC and nontuberculous mycobacterial organisms. Endoscopy equipment can be similarly infected during cleaning. Despite the widespread nature of MAC organisms in the environment, infections in dogs and cats have been relatively uncommon owing to their innate resistance. Similarly unusual in exotic carnivores, a single instance of disseminated M. avium infection in a captive tiger (P. tigris) was associated with feeding of infected raw chicken.54 In contrast, poultry and swine are commonly susceptible to MAC infection after contact with infected food or water. In contrast to the classical tuberculous and lepromatous lesions described for humans with mycobacteriosis, cats with mucocutaneous infections caused by M. tuberculosis or M. avium–complex organisms develop a pyogranulomatous infiltrate with variations in the amount of necrosis, presence of multinucleated giant cells, and degrees of lymphoid infiltration.173 The M. tuberculosis organisms are frequently extracellular, whereas the M. avium–complex organisms are usually intracellular. The pattern of inflammation does not specifically correlate with the causative organism and in some instances, it can resemble that of atypical cutaneous mycobacteriosis. The cytokine staining pattern of feline mucocutaneous mycobacteriosis is similar to that described for human leprosy in which T-cell–mediated response leads to epithelioid granulomas with abundant CD4+ type 1 helper T (Th1) cells and upregulation of the cytokines interleukin (IL)-1b, tumor necrosis factor-α, IL-6, and granulocyte-macrophage colony-stimulating factor.173 The factors that contribute to mycobacterial resistance by the host are unclear.154 CMI is typically associated with protection against facultative intracellular pathogens such as mycobacteria. Increased resistance seems to be associated with the enhanced capacity of activated macrophages to kill tubercle bacilli or to inhibit their intracellular multiplication. In people with refractory pulmonary mycobacteriosis caused by non–M. tuberculosis organisms, a defect in interferon (IFN)-γ secretion has been documented.270 Infection with MAC organisms usually begins with ingestion of the organism from the environment or contaminated food or uncooked infected viscera. MAC organisms in dogs and cats have often been disseminated throughout lymphoid and many other tissues, without indications of a primary granuloma at the site of entry. These organisms are closely related to M. avium subspecies paratuberculosis, the cause of Johne’s disease, which is a chronic granulomatous enteritis of ruminants and other herbivores. Animals with Johne’s disease may have acquired the infection as neonates, through eating contaminated food or exposure to a contaminated environment. The mycobacteria may be phagocytized by intestinal macrophages, at which time the infection becomes quiescent. Eventually, with stress or acquired or inborn immunosuppression, the organisms replicate and the disease occurs. Similar events are suspected to occur in MAC infections of the predisposed dog or cat breeds, which develop disseminated infections within the first few years of life, likely as the result of unrecognized defects in CMI. Basset hounds and miniature schnauzer dogs, and Siamese and Abyssinian cats, are overrepresented in reports of MAC infection. Siamese cats are similarly predisposed to infections with other persistent intracellular organisms, and Abyssinian cats have immune aberrations resulting in a high prevalence of reactive amyloidosis. Although defects in the immune system of these breeds are likely, the precise nature of these defects have not been completely determined (see Chapter 94). No clear association has been made between feline retroviral infection and mycobacteriosis. In some animals with severe immunosuppression, bacteremia may result and multiple organ dissemination ensues. Table 48-2 reviews the clinical features of disease caused by the various mycobacterial species. TABLE 48-2 Comparison of Species of Mycobacterium Infecting Dogs and Cats
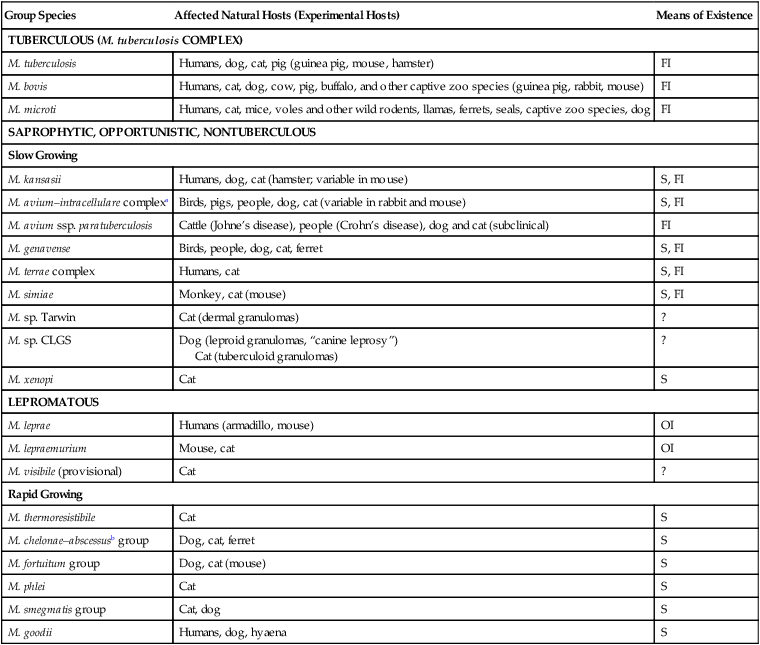
Mycobacterial Infections
Group Species
Affected Natural Hosts (Experimental Hosts)
Means of Existence
TUBERCULOUS (M. tuberculosis COMPLEX)
M. tuberculosis
Humans, dog, cat, pig (guinea pig, mouse, hamster)
FI
M. bovis
Humans, cat, dog, cow, pig, buffalo, and other captive zoo species (guinea pig, rabbit, mouse)
FI
M. microti
Humans, cat, mice, voles and other wild rodents, llamas, ferrets, seals, captive zoo species, dog
FI
SAPROPHYTIC, OPPORTUNISTIC, NONTUBERCULOUS
Slow Growing
M. kansasii
Humans, dog, cat (hamster; variable in mouse)
S, FI
M. avium–intracellulare complexa
Birds, pigs, people, dog, cat (variable in rabbit and mouse)
S, FI
M. avium ssp. paratuberculosis
Cattle (Johne’s disease), people (Crohn’s disease), dog and cat (subclinical)
FI
M. genavense
Birds, people, dog, cat, ferret
S, FI
M. terrae complex
Humans, cat
S, FI
M. simiae
Monkey, cat (mouse)
S, FI
M. sp. Tarwin
Cat (dermal granulomas)
?
M. sp. CLGS
Dog (leproid granulomas, “canine leprosy”)
Cat (tuberculoid granulomas)
?
M. xenopi
Cat
S
LEPROMATOUS
M. leprae
Humans (armadillo, mouse)
OI
M. lepraemurium
Mouse, cat
OI
M. visibile (provisional)
Cat
?
Rapid Growing
M. thermoresistibile
Cat
S
M. chelonae–abscessusb group
Dog, cat, ferret
S
M. fortuitum group
Dog, cat (mouse)
S
M. phlei
Cat
S
M. smegmatis group
Cat, dog
S
M. goodii
Humans, dog, hyaena
S
Infections Caused by Slow-Growing Mycobacteria
Etiology
Tuberculous Mycobacteria
Nontuberculous Mycobacteria
Mycobacterium avium Complex
Epidemiology
Tuberculous Mycobacteria
Mycobacterium tuberculosis
Mycobacterium bovis
Mycobacterium microti
Nontuberculous Mycobacteria
Mycobacterium avium Complex
Pathogenesis
Tuberculous Infections
Nontuberculous Infections
Clinical Findings
Organism
Environmental Factors
Clinical Features
Drug Susceptibility or Reported Successful Therapya
SLOW-GROWING TUBERCULOUS: TUBERCLES AND LYMPHADENITIS, OCCASIONAL DISSEMINATION
M. tuberculosis
Urban, close contact with affected person
Usually respiratory, pulmonary localization, can disseminate systemically
Isoniazid, rifampin, ethambutol, pyrazinamide
M. bovis
Rural cats, ingest raw beef or dairy products or infected wildlife
Usually alimentary disorders; may get respiratory, cutaneous, or lymphatic involvement, sometimes systemic dissemination
Rifampin, clarithromycin, quinolones, ethambutol, isoniazid, surgical excision of skin lesions
M. microti
Rural, suburban, hunter, bite wounds, prey exposure, ingestion of rodents
Nodular cutaneous lesions draining, ulceration, peripheral lymphadenomegaly, local myositis, arthritis, osteomyelitis, sometimes pneumonia, peritoneal infection, or systemic dissemination
Clarithromycin/azithromycin, quinolones + rifampin; rifampin, isoniazid, ethambutol
LEPROMATOUS: CUTANEOUS NODULAR DERMATOSIS
M. lepraemurium
Cooler wet climates, winter months, cats under 3 yr of age exposed to infected rodent prey
Single to multiple cutaneous and subcutaneous dermal nodules on head and extremities, ulcers, fistulas, abscesses regional spread only
Clofazimine, clarithromycin, doxycycline or minocycline, rifampin, surgical removal
Feline leprosy, M. sp. Tarwin
Central coast New South Wales, Australia, New Zealand, older cats over 10 yr of age, feline immunodeficiency virus predisposes
Multiple subcutaneous dermal nodules, no ulceration, sometimes dissemination
Clarithromycin, rifampin, clofazimine
“Candidatus M. visibile”
Environmental exposure?
Cutaneous and disseminated
Clofazimine
NONTUBERCULOUS: PYOGRANULOMATOUS
Saprophytic Slow Growing: Cutaneous Lesions, Lymphadenitis, Dissemination in Immunocompromised Hosts
M. avium complex
Exposure to infected soil, water or dust; acidic soils contaminated with bird feces or carcasses, basset hounds and Siamese and Abyssinian cats most prevalent
Dermal and regional lymph node granulomas, alimentary infiltration, corneal granulomas, systemic dissemination
Clarithromycin, clofazimine, doxycycline or minocycline, rifabutin, ethambutol; rifampin preferred if central nervous system involvement for better penetration
M. genavense
Environmental exposure in immunocompromised host
Disseminated lymphadenitis
Clarithromycin, ethambutol, quinolones, clofazimine
M. terrae complex
Environmental exposure
Cutaneous lesions
Clarithromycin, quinolones, rifampin
M. simiae
Environmental exposure
Cutaneous and disseminated
Clarithromycin, quinolones, rifampin?
M. ulcerans
Environmental exposure
Cutaneous
Surgical removal, clarithromycin
Canine leproid granuloma, M. sp.
Probably worldwide, biting flies
Subcutaneous nodules especially on the head and ears
Surgical removal, rifampin, clarithromycin
Saprophytic Fast Growing: Cutaneous and Subcutaneous Pyogranulomatous Infections
M. thermoresistibile
Soil and house dust, inhaled water, wound contaminant
Pyogranulomatous pneumonia, pyothorax, cutaneous and subcutaneous pyogranulomas
Doxycycline, clarithromycin
Other fast-growing opportunistic species
Soil and water exposure; bite and puncture wounds; immunocompromised host
Cutaneous and subcutaneous granulomas, especially inguinal region, ulcers, drainage, with regional spread only; secondary wound infections
Surgical removal, wide excision, variable susceptibility to quinolones, doxycycline, aminoglycosides, clofazimine, clarithromycin, trimethoprim-sulfonamide ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Mycobacterial Infections