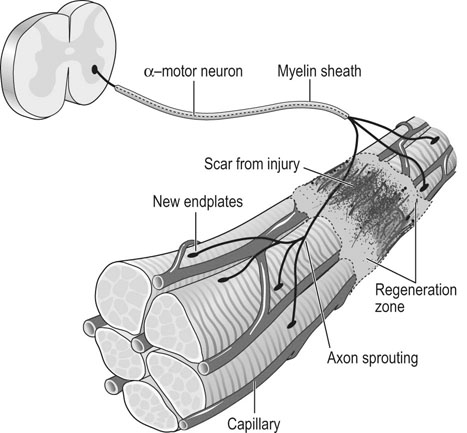
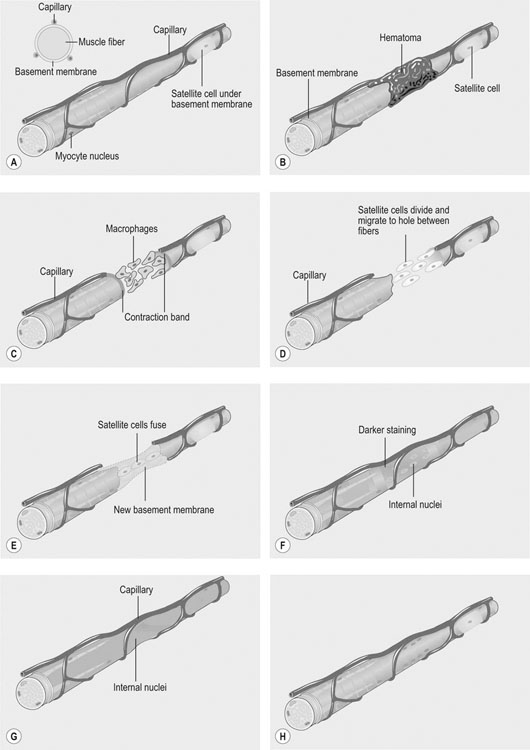
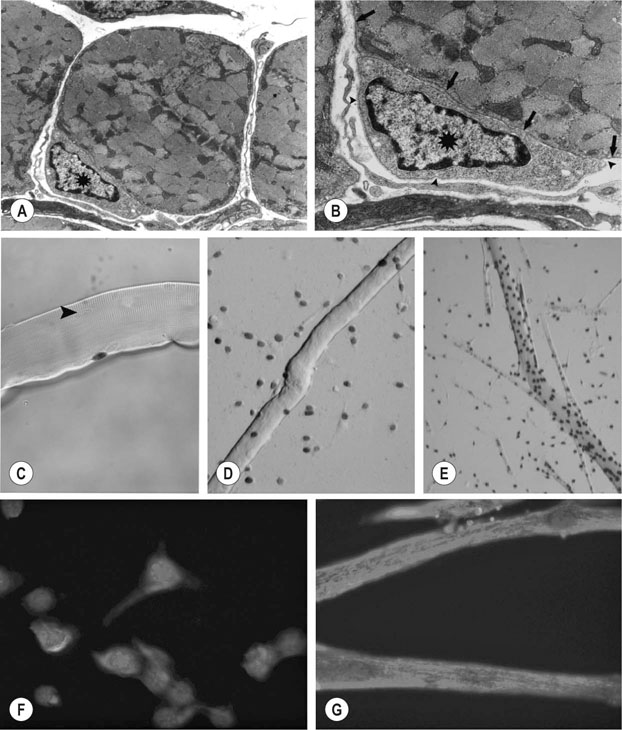
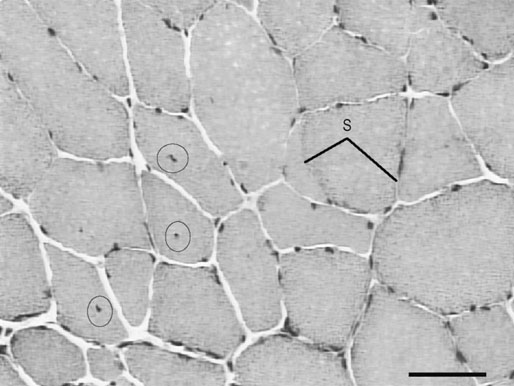
• recognition of similarities between human and equine muscle diseases • greater awareness that muscle has a limited range of responses despite different mechanisms of injury • subdivision of diseases based on breed susceptibility and histopathology, and • the application of genomic, genetic, molecular and cellular investigative techniques. Future developments should lead to more accurate diagnostic tests and better treatments, thereby enabling the veterinarian to provide an improved service, while benefiting from a more clinically rewarding experience. Muscle tissue has a limited range of responses to different insults whether traumatic, ischemic, exercise-induced or due to underlying disease. Cell membrane damage causes abnormal ion fluxes and osmotic imbalance that rapidly perturbs fiber homeostasis. Normally the resting myoplasmic Ca2+ concentration is maintained at a concentration that is 60–100 times lower than that of the extracellular fluid (Chapter 6). Membrane damage allows Ca2+ to enter the cytoplasm from the interstitium, causing activation of destructive cellular proteases and inhibition of mitochondrial respiration.1,2 Necrotic cell death is often associated with inflammatory responses including the chemotaxis of neutrophils and macrophages and collagen deposition. However, in certain muscle diseases, fibers die without marked inflammatory responses in which case cell death can be due to apoptosis.3 Muscle’s remarkable regenerative capacity is usually associated with a characteristic sequence (Fig. 7.1). Following injury, ruptured myofibers retract, forming a gap between the stumps and allowing access for inflammatory cells via capillaries.4 Contraction bands – condensations of cytoskeletal and sarcomeric material – plug the myofiber stumps and prevent further damage prior to plasma membrane repair.5,6 Satellite cells, a subpopulation of adult skeletal muscle stem cells7 that are normally relatively undifferentiated, quiescent and located between the myofiber’s sarcolemma and its basement membrane (Fig. 7.2), are responsible for regeneration. Growth factors cause satellite cell activation, division and differentiation within 24 hours.8 The cells migrate into the damaged region and over a period of about five days, fuse to form multinucleated myotubes (often within the basal lamina of the damaged fiber).9 As the proteins of the myofilaments mature, myotubes gradually differentiate into myofibers,5 a process taking several months, during which histopathology reveals immature fibers of variable sizes and with internally-located nuclei (Fig. 7.3).10 Basement membrane damage results in more extensive fibrosis that can impede reinnervation and revascularization and hence regeneration.9 Scarring might hinder action potential proliferation; however, this is overcome by sprouts from nearby axons piercing interposed scar tissue and creating new neuromuscular junctions (Fig. 7.4).9 • Muscle damage caused by strains or tears is common in athletic horses. • The site of damage dictates the signs that can include lameness or back stiffness. • Plasma CK and AST activities can be mildly elevated. • Ultrasound, thermography and scintigraphy can aid diagnosis. • Rest and non-steroidal anti-inflammatory drugs often bring full return to function. Delay in the onset of inflammation after trauma might complicate the history, but pain can be evident in showjumpers and eventers (both prone to lumbar and gluteal strain) as unwillingness to jump or turn sharply11 or in other animals as mild to moderate lameness or stiffness. Generally, injuries occur during exercise but severe muscle damage can follow prolonged recumbency or direct trauma.12–14 In acute stages, palpation reveals painful muscles with focal heat, swelling and edema. Trauma, secondary to impact injury, can be associated with overlying skin damage. In more chronic cases, an organizing hematoma, a scar, or both might be palpable. Certain lacerations and muscle tears can manifest as an abnormally held limb, unusual gait or an inability to bear weight.14,15 • Both acquired and inherited forms of exertional rhabdomyolysis exist. • Disease severity ranges from subclinical to life threatening. • During an episode, plasma CK and AST activities are moderately to markedly elevated but they can return to normal between episodes. • In severe cases, myoglobinuria can cause renal tubular damage and acute renal failure. • In acute, severe cases, rest and intravenously-administered isotonic fluids and analgesics form the mainstays of treatment. • Depending on breed, muscle biopsy, genetic testing, or both are indicated in an animal with a history of multiple episodes. • Overexertion is likely a common cause of acquired disease. • A common genetic cause in multiple breeds, known as polysaccharide storage myopathy type 1 (PSSM1), is associated with an autosomal dominant, gain of function mutation in the skeletal muscle glycogen synthase gene. • Separate, but likely inherited disorder(s), occur in Thoroughbreds, Standardbreds and several other breeds. • Environmental influences probably modify the phenotype in genetically-susceptible animals. • Dietary and exercise management can prevent recurrence in many horses. There are many historical, somewhat speculative reports suggesting different possible causes of equine exertional rhabdomyolysis (ER).20 A large number of causes of this disease in horses is unsurprising given the numerous acquired and inherited forms in humans.21,22 However, since certain types of equine ER have underlying genetic causes, the intermittent and varying severity of phenotype in these animals can be explained by the influence of modifying genes and environmental factors; factors that in the past were determined to be the primary etiology (Fig. 7.5). Episodes of rhabdomyolysis not generally associated with exercise can be of toxic, infectious, immune-mediated or iatrogenic origin (Fig. 7.5) and are not included in this chapter but are discussed in detail in reviews or other medicine texts.23 In horses with acute exertional rhabdomyolysis, often the history includes recent exercise and sometimes training or management changes. The complaint can vary from a mild, stilted gait to severe stiffness, sweating or recumbency.24 However, most animals are only mildly or moderately affected. Some horses will present as apparently clinically normal, but with histories compatible with recurring episodes of post-exercise stiffness or gait changes, that might previously have been classified as muscle-related by appropriate biochemical testing. Frequently, there is no good explanation for the rhabdomyolysis and owners believe that some underlying factor is responsible. Some horses present with histories of poor performance.25 Although most horses are clinically normal between episodes, during an episode horses can show varying clinical signs. Mildly or moderately affected animals are tachycardic, with firm, painful pelvic limb, epaxial and gluteal musculature causing gait stiffness.24 In some, pain localization can be difficult or pain is manifest in other ways: male horses, for example, sometimes posture to urinate26 and other horses exhibit colic. Pigmenturia might be evident, especially in more severe cases (Fig. 7.6). These animals are often extremely painful, tachycardic, hyperthermic and tachypneic; they sweat profusely and can be totally unwilling or unable to move.24 These horses have widespread muscle involvement and can become recumbent. The worst affected animals can show signs compatible with underlying shock and disseminated intravascular coagulation. Routine clinicopathologic changes in mild cases, during or immediately following an episode, usually consist solely of elevations in the plasma activities of the muscle-derived enzymes creatine kinase (CK) and aspartate aminotransferase (AST). Enzyme activity can be normal in horses between disease episodes, even when significant underlying muscle lesions are present.27 Plasma CK activity is the most convenient and specific marker of acute muscle damage and peaks at 4–6 hours following muscle damage and (unless the damage continues) starts to decline, with a half-life of approximately 12 hours (Fig. 7.7).28 AST activity peaks about 24 hours after an episode and can remain elevated for several days to weeks.13,26 Although both CK and AST activities rise in proportion to the degree of muscle damage, they do not always reflect the severity as assessed clinically or the prognosis. A high plasma AST activity without a concomitant elevation in plasma CK activity can indicate that muscle damage has occurred within preceding weeks. However, given that AST is not a specific marker for muscle disease, careful evaluation of the hemogram and biochemistry profile, and if necessary, further tests, are indicated to rule out hepatocellular disease. During disease episodes, more severe cases have additional less-specific abnormalities. Hyperkalemia can reflect the release of potassium from damaged muscle fibers. The hematocrit and total protein can rise due to intracompartmental fluid shifts. High serum creatinine concentration suggests the possibility of acute renal failure. Complex acid-base abnormalities are sometimes present as the usual hypochloremic metabolic alkalosis29 shifts to metabolic acidosis if shock ensues. Widespread hematological and biochemical abnormalities are evident in terminal cases. Ideally, a positive test should provoke a subclinical episode of rhabdomyolysis that can be detected via a rise in CK activity between pre- and 4h post-exercise serum samples or in AST activity between pre- and 24h samples. Titrating the amount and type of exercise can be difficult, but should be based on the horse’s history and level of fitness. Bouts of maximal exercise appear less likely to precipitate episodes in Thoroughbreds with recurrent exertional rhabdomyolysis (RER),30,31 and generally, 10–20 minutes of moderate exercise (trot and canter) on a longe line or a treadmill is considered appropriate. The sensitivity and specificity of exercise tests have not been evaluated however and the intermittent nature of the disease can result in a negative test in a susceptible animal. Furthermore, it remains unclear whether the same exercise testing protocol is appropriate for identification of all forms of ER since types or duration of exercise have not been compared in horses with different underlying disorders. Disparity among horses with results of exercise testing is revealed by studies in different breeds with the same underlying disorder: the rise in serum CK activity detected in Belgian and Percheron draught horses with PSSM1 after moderate exercise was greater in homozygotes than heterozygotes but there was considerable overlap between the groups (range of CK rise in both groups 21% to 826%).27 In a separate study, the mean rise in CK activity in exercised Haflinger heterozygotes with PSSM1 was approximately 40%.32 Importantly, in neither study was there a rise in CK or AST activity after exercise in unaffected horses.27,32 Consequently, even modest rises in CK or AST after exercise should be considered as potentially relevant. A urine sample collected early following a disease episode might, with microscopic examination, reveal urinary casts, a useful indicator of tubular necrosis and impending acute renal failure prior to the plasma creatinine concentration rising. Reagent strip analysis of urine does not differentiate myoglobin from hemoglobin, so specific assays are required to determine the cause of any pigmenturia. However, measurement of urinary myoglobin concentration is not usually necessary in an animal with significantly elevated serum muscle enzyme activities. Mild pigmenturia is a normal finding in some horses following high-intensity exercise.33 Urinary clearance ratios are calculated to assess whole-body electrolyte status and renal function and historically, were used to evaluate horses with histories of ER.34 Electrolytes and creatinine concentrations are measured in a urine (free catch or catheterized) and serum sample from the same animal. Ratios determined following collection of a single urine sample give similar results to those obtained with a 24-hour pooled (volumetric) urine sample, except for magnesium,35 but accurate measurement requires urinary acidification (e.g. addition of concentrated nitric or hydrochloric acid)35,36 to dissolve any suspended crystals. Horses should not eat between collection of the samples.37 The fractional clearance for each electrolyte (FC(electrolyte)), is calculated as follows:38 where the square brackets represent the concentration (the acidification dilution factor should be factored in). There are wide variations for normal values in veterinary literature,36,38–41 probably from differences in management, sampling and analysis. Normal ranges for FC(sodium) have been reported as 0.04–0.52%; for potassium, 35–80%; for chloride, 0.7–2.1%; and for phosphate, 0–0.2%.34 The calculation of electrolyte clearance ratios during an episode of ER can help evaluate renal function,38 but should not be used to determine whether electrolyte imbalances were responsible for precipitating the attack. Furthermore, identification of wide daily fluctuations in clearance ratios in the same horse, despite standardized management, casts serious doubt on the test’s interpretation or relevance.39,41 Depending on the breed (see genetic testing below), muscle biopsy is often indicated in an animal with several unexplained episodes of ER (Fig. 7.8). The closed Bergstrom technique is described in Chapter 6; however although this method is useful in the research setting where samples can be handled rapidly, it is often preferable to collect an open biopsy in the clinical setting, since larger samples can be obtained that are less prone to drying artifact. The biopsy site is based on the physical examination, but epaxial, gluteal and semimembranosus muscles are most commonly chosen for exertional myopathies.42 Ideally, a fresh muscle sample should be snap-frozen immediately in isopentane cooled in liquid nitrogen,10 but since this is not usually practical, a compromise is necessary. Good results can be obtained when a sample is sent overnight to a specialist laboratory provided it is packaged appropriately.43 Details regarding an appropriate sampling method follow in Box 7.1. Histopathological examination and interpretation is compromised in samples that are exposed to excessive saline or that are submitted at ambient temperature;43consequently samples are best submitted in a sealed dry plastic container, without saline-moistened gauzes, and kept chilled (not frozen) on ice packs and sent in a polystyrene (Styrofoam) box. Formalin fixation, though more convenient, is unsuitable for histochemical investigation and leads to more artifact;44 it does allow morphological assessment however, and has enabled a diagnosis to be reached in cases of polysaccharide storage myopathy (PSSM).45 Genetic testing is available for detection of specific mutations associated with PSSM1 and malignant hyperthermia (MH) (see sections on etiology). Blood samples (in EDTA) or hair plucks containing the roots should be submitted to specialist laboratories. Since pretest probability for disease-associated mutations is higher in certain breeds, genetic testing is recommended before more invasive testing (for example muscle biopsy) in some animals (Fig.7.8). The history, clinical signs and clinicopathologic investigation (see below) are usually sufficient to establish a diagnosis of ER but scintigraphy can be helpful to localize muscle involvement in certain cases.16 Alternatively scintigraphy can suggest an underlying muscle problem where instead a skeletal disorder was suspected (Fig.7.9). Additional tests are often recommended in human patients with unexplained ER. Depending on the clinical presentation, and histopathology, these can include specific enzyme histochemistry or immunohistochemistry, electron microscopy, in vitro contracture testing of muscle strips, assessment of urine organic acid concentrations, analysis of plasma acyl carnitine profiles, measurement of plasma lactate and pyruvate concentrations before and after exercise, respiratory chain complex activity measurement, testing for fatty acid oxidation defects or specific DNA sequencing. Clinicians should contact a specialist laboratory to discuss more advanced testing if routine investigation has previously been unrewarding or if there has not been a good response to treatment or management changes (Fig. 7.8). Histopathological assessment often reveals widespread muscle involvement, even in non-locomotor muscles. Occasionally there can be focal muscle involvement so a wide selection of muscles should be sampled, including several epaxial muscles, thoracic and pelvic limb locomotor muscles and psoas muscle. Lesions in muscle can indicate acute disease or provide evidence of an ongoing or chronic underlying problem (see sections on pathology below). Unfortunately, formalin fixation of muscle substantially limits the histopathological examination and any future diagnostic modalities. In the acute form, ER syndrome can be confused with colic, laminitis, tetanus, hyperkalemic periodic paralysis and some cardiac arrhythmias. Usually these disorders are readily distinguishable by additional signs, specific tests and the lack of significantly elevated serum muscle enzyme activities. However, in animals with raised CK or AST activities, it is important not to overlook the possibility of two separate (unrelated) disorders occurring in the same animal, given the high prevalence of specific forms of ER within certain breeds. For example, some forms of ER are more likely encountered following time off, or during investigation for a separate injury or disease: in these cases, clinical judgment is necessary to determine the primary, clinically-relevant problem. Similarly, coincidence, rather than direct association, was proposed as an explanation for a previously suggested,46 but now refuted, link between Shivers syndrome and PSSM in susceptible animals.47 Occasionally, sedentary horses present with classic signs of rhabdomyolysis with markedly elevated serum muscle enzyme activities. In these animals, underlying genetic susceptibility more usually associated with exertional forms of the disease is possible, because other events such as stress can precipitate attacks. Toxic, infectious or immune-mediated muscle diseases should also be considered.23,42,48,49 In an animal with acute, ongoing rhabdomyolysis, the therapeutic goals are to minimize further muscle damage, to prevent myoglobin-induced kidney injury by establishing and maintaining diuresis, to correct underlying systemic metabolic or cardiovascular abnormalities and provide analgesia. Early and prompt treatment is essential to maximize the chances of recovery.50 Mildly affected animals – when vital signs are close to normal – can recover from an acute episode without intravenous administration of isotonic fluids. However, they should be monitored for signs of deterioration. In moderate to more severe cases, however (even in those without pigmenturia), establishing diuresis and preventing or treating hypovolemia is the priority because myoglobin is nephrotoxic. Large volumes of isotonic fluids are usually effective (0.9% NaCl or lactated Ringer’s solution infused intravenously at 100–150mL/kg/24h). Urine should be alkalinized, however, the addition of sodium bicarbonate to fluids is rarely necessary,29 and only indicated in a horse with metabolic acidosis when the urine remains acidic despite fluid therapy, because myoglobin is significantly more nephrotoxic when in acidic urine.51 Dantrolene, a drug that limits release of Ca2+ from the sarcoplasmic reticulum (SR) by antagonising the skeletal muscle ryanodine receptor (RYR1),52 has been used in the acute stages of idiopathic rhabdomyolysis53 but pharmacokinetic studies54,55 suggest that the dose and the frequency of administration were unlikely to have resulted in beneficial drug concentrations based on those required to effect a response in humans.56 Refer to the section on prevention (below) for a discussion of use of dantrolene for ER prophylaxis. The long term prognosis for successful athletic function varies, depending on the etiology and whether appropriate management and prophylaxis are instituted. Future athletic function can be compromised in animals with substantial underlying muscle lesions, however many horses with inherited forms of ER compete successfully: indeed evidence suggests that certain forms of ER are associated with underlying improved athletic performance.57 The association of a disease with a considerable number of anecdotal preventative treatments usually reflects the treatments’ questionable or limited efficacies and current and historical inability to establish the precise etiology. Such is the case for the prophylaxis of ER where a wide variety of putative preventive treatments exists,58 with only a few having been scrutinized objectively. Much of this confusion likely relates to the fact that ER in horses has multiple etiologies and that therefore one treatment is unlikely to be effective for all forms of the disorder. Despite apparent differences in etiology and pathogenesis,59 the substitution of a proportion of dietary calories derived from soluble carbohydrate, with additional fat, reduces the severity of episodes of ER via poorly understood mechanisms in both Thoroughbreds with RER60 and horses with PSSM1.61 High-carbohydrate diets lead to more severe muscle damage in horses with RER,30,60 while high-fat diets are protective.60 Though unexplained, one possibility is that a high-fat diet can result in the stabilization of sarcolemmal membranes but the rapidity of the protective response60 suggests that this is unlikely. High-fat diets have a calming effect on horses62 and are associated with lower plasma cortisol concentrations during exercise.63 Since stress has been associated with RER in Thoroughbreds,58 this dietary calming effect could explain the rapid prophylactic efficacy of high-fat diets in RER.64 Stress is also associated with ER in Standardbred horses:57 consequently, though not yet tested experimentally, similar dietary adjustment might also be beneficial in this breed. There is conflicting evidence as to whether low-carbohydrate (high-fat) diets reduce the excessive glycogen accumulation in muscle in horses with PSSM.65,66 However, since glycogen synthase activity (and therefore glycogen synthesis) in muscle is increased by glucose-6 phosphate,67 which itself rises in muscle during hyperglycemia,68 diets high in soluble carbohydrates and simple sugars are probably best avoided in horses with PSSM1. Dietary adjustments of this kind reduce the severity of myopathy in PSSM1-affected animals.69 Most studies have investigated the beneficial effect of diets that contain approximately 20% fat, together with a reduction in soluble carbohydrate (grain) intake.60 Increased fat in the diet can be achieved by the addition of vegetable oil (up to approximately 1g/kg bodyweight per day or 1.1mL/kg bodyweight per day). Rice bran (15–20% fat) can also be used as a substitute source in animals that find the oil unpalatable or a combination can be suitable in some animals.70 Forage intake should be at least 1% of bodyweight, but fast-growing lush pastures and high-quality sweet hays should be avoided in horses with PSSM1.71 Horses should be introduced to higher fat diets over several weeks and the dietary intake of minerals and vitamins should meet current recommendations.72 In particular, owners should ensure that the calcium: phosphorus ratio in the diet is adequate, as rice bran and high-fiber products contain excessive phosphorus relative to calcium. There are several high-fat, low soluble-carbohydrate feeds that are commercially available. Unfortunately, trainer compliance within racing yards is sometimes hard to achieve, because of concern that Thoroughbred horses in training lose condition on diets designed for management of ER. Finally, it is important to remember that the efficacy of dietary adjustment has only been established in RER and PSSM1 and that horses with other forms of ER, or idiopathic disorders, might not respond. Indeed, in ER-affected humans with fatty acid metabolism defects, low fat and high carbohydrate diets are sometimes recommended.73 Consequently, further diagnostic investigation might be warranted in horses that do not respond as expected to widely-accepted dietary changes. Although electrolyte supplementation is appropriate in animals that have been identified as deficient by specific testing, it is unlikely to be of benefit in most horses. There is no valid rationale for the once popular administration of sodium bicarbonate to Thoroughbred horses with RER to prevent episodes, because most affected animals do not have underlying acid-base disorders prior to exercise and become alkalotic during exercise.29,60 Although dantrolene administration has been associated with causing paresis in some horses at high doses (4mg/kg IV54 and 9mg/kg PO,75) manifest as muscle trembling or prolonged recumbency, some experimental evidence76,77 supports its use in RER prophylaxis in Thoroughbred horses. As it has a short half life, administration should be 1.7–4 hours before exercise to ensure peak plasma concentrations at the time of exercise;54,55 published and anecdotal reports suggest that a dose of 1–3 mg/kg PO)76 can be beneficial. An early report suggested that feed restriction is necessary to optimize bioavailability,76 however more recent data suggests that feed withdrawal is unnecessary: indeed it can adversely affect drug absorption.78 A low (approximately 1mg/kg) dose was undetectable in horse urine after 168 hours (7 days).55 Oral dantrolene, as prophylaxis for ER has not been tested in breeds other than Thorougbreds, or in disorders other then RER, but epidemiological evidence suggests that it could be beneficial, particularly in breeds where the disease etiology is suspected to be similar or identical to the Thoroughbred disorder, RER.57 Studies reveal that vitamin E or selenium supplementation enhances plasma anti-oxidant capacity and can reduce oxidative stress79 but there is no good evidence that either can reduce exercise-associated increases in CK or AST activity, or the frequency or severity of episodes of ER:80,81 There is evidence that supports an owner placebo effect, rather than any beneficial effect of anti-oxidant supplementation in horses with various perceived muscle-related problems.82 Despite this, vitamin E (1–6 IU/kg/day alpha-tocopherol) and selenium (1–2mg/day) supplementation in food are probably indicated when deficiencies have been confirmed. Although reaching a diagnosis of ER is not usually difficult, categorizing the disease according to the etiology can be much harder. Both acquired and inherited causes should be considered in an animal with acute signs whereas in a horse that presents following multiple episodes, underlying genetic predisposition is more likely. Certain acquired causes can be obvious from the history (e.g. overexertion) and specific genetic testing can identify other forms. Unfortunately, many cases contribute to a large idiopathic category, a situation in common with the diagnosis of human ER.21,22 Continued use of muscle biopsy, other tests, and more research is likely better to categorize ER-associated disorders in horses in the future. Delayed-onset muscle soreness in humans is associated with damage caused by excessive eccentric contractions (contraction during muscle lengthening).84 Stiffness and pain, usually experienced 1–2 days following such exercise, is initially most evident at the myotendinous junction and then spreads throughout the muscle. Although the mechanism is poorly understood, there are prominent signs of damage within a muscle following eccentric contractions that include disruption of sarcomeres and damage to the excitation-contraction coupling mechanism and the sarcolemma.85 This damage is reflected by (sometimes considerable) elevations in serum muscle enzyme activities86,87 and triggers a local inflammatory response accompanied by edema and the sensitization of nociceptors.84 Whether horses experience this syndrome is unknown. Exercising horses are most likely to encounter eccentric contractions during downhill exercise and jumping. Given that muscle damage in humans generally occurs when the type or extent of eccentric contractions are unaccustomed and that adaptation occurs with training,85 it seems sensible to introduce horses gradually to these types of exercise. Exceeding the level of training either by excessive endurance exercise or overexertion when galloping is a common cause of acute ER in horses.88 Exhaustion during endurance exercise results in heat retention, fluid and electrolyte loss, acid-base imbalance and intramuscular glycogen depletion.89 Although the causes are numerous and likely involve electrolyte imbalances and hyperthermia, in certain cases a presumed underlying factor is the deficiency of ATP, which results in an inability to maintain ion homeostasis. In turn, a corresponding rise in intracellular calcium concentration precedes the final common pathways, leading to muscle fiber death described at the beginning of this chapter. Increased oxygen utilized during exercise leads to proportionate increases in free radical production (Fig. 7.10).90 Free radicals are widely believed to cause post-exercise stiffness and fatigue in muscle, through several deleterious mechanisms that include the peroxidation of lipid membranes. Cell damage is normally minimized by the action of a complex cascade of free radical scavengers and antioxidants, including vitamin E and the selenium-dependent enzyme, glutathione peroxidase. When antioxidants fail to quench free radicals sufficiently, the body is subjected to so-called ‘oxidative stress’.90 Exercise-induced oxidative stress occurs in horses, particularly when ambient temperature and humidity are high,91but most studies fail to provide evidence supporting a role for oxidative stress in exercise-associated sarcolemmal damage.80,81,92 Furthermore, if oxidant damage played an important role in horses with ER, one might expect deficiencies (through excessive consumption) to be encountered commonly in ER-susceptible animals, but this is not the case in either ER-prone Arabians93 or Belgian horses with PSSM1.27 Indeed, in one study, higher serum and muscle vitamin E concentrations and serum glutathione peroxidase activities were detected in ER-susceptible Standardbreds compared with controls, although supplementation could not be ruled out as a cause of these higher concentrations.94 In adult horses, vitamin E or selenium deficiency has been associated with nutritional myodegeneration (white muscle disease) of the masseter muscle95 and was implicated in horses with rhabdomyolysis, colic and myocardial disease.96 In growing foals, nutritional myodegeneration is characterized histopathologically by changes that are sometimes seen in horses with ER.97 However, such changes likely reflect the general response of muscle to cycles of degeneration and regeneration, rather than damage caused specifically by oxidative stress. Published and anecdotal reports of improvement following correction of electrolyte clearance ratios underlie historical interest in this area.34 In a group of 38 ER-susceptible Thoroughbreds, about a third had potassium clearance ratios of less than 30% or low chloride clearance.98 These differences could potentially reflect differences in the handling of electrolytes by ER-susceptible animals but Thoroughbred horses prone to ER exhibit the same dietary-induced alterations to electrolyte clearance ratios as normal animals.35 Much attention has been directed at potassium, because low muscle potassium concentrations can precipitate rhabdomyolysis in other species.99 Erythrocyte potassium concentration has been measured in attempts to investigate whole-body potassium stores: results indicate both low100 and normal98 erythrocyte potassium in horses with ER. However, the significance of either study is questionable given that erythrocyte potassium concentrations do not correlate with muscle or plasma potassium concentrations in horses.98,101,102 Compared with normal animals, lower dry weight muscle potassium concentrations were detected in ER-susceptible horses.98 However, characteristic muscle histopathological changes, seen in humans with potassium deficiency,99 are generally not observed in muscle from horses with ER so it is hard to draw conclusions about the importance of potassium in causing or exacerbating ER in horses. The primary influence of altered electrolyte status as a cause of ER is better studied prospectively in normal horses, rather than retrospectively in horses known to be susceptible to the disorder. In comparison with controls, normal Thoroughbreds administered furosemide (frusemide) and sodium bicarbonate developed lower plasma calcium, chloride, magnesium and potassium concentrations. Following exercise, serum CK activity was significantly higher in the treated group,103 though results were skewed by two of the six animals, that could have had an underlying primary genetic susceptibility. Consequently, as with other factors, it is possible that electrolyte imbalance can modify the phenotype of a genetically-susceptible animal. In experimental animal models, sex hormones influence the degree of disruption and inflammatory response to injury in skeletal muscle. For instance, estrogen protects muscle from injury induced by eccentric contractions and ischemia-reperfusion injury.104 It is intriguing therefore that many studies of RER report a higher incidence in female horses compared with males.57,58,105–109 No correlation has been found between the stage of the estrus cycle and plasma CK activities in Thoroughbreds in training,110 suggesting that a direct association of female sex hormones with ER is unlikely. As yet, therefore, the higher incidence of ER in females remains unexplained. No association with sex is seen in PSSM1 (see below).61 Hypothyroidism is associated with subclinical elevations of serum CK activity and rarely causes ER in humans.111,112 Although a cause of poor performance in horses,113 hypothyroidism has not been associated with ER in horses.59,114–116 Other signs associated with hypothyroidism in horses, such as alopecia, lethargy and excessive fat accumulation,117,118 are not typical of ER-susceptible horses and, given its rarity, it is unlikely that hypothyroidism is associated with ER in most animals. Equine herpes virus 1 (EHV1) infection was proposed as causing an outbreak of ER in a training yard, where several horses seroconverted to the virus.119 Equine influenza virus (EIV) has also been diagnosed by seroconversion as the potential cause of certain equine myopathies.106 Both EIV and EHV1 infections are common in groups of young race horses and although the acute-phase response to viral infection can cause transient arthralgia and myalgia, and therefore stiffness, rhabdomyolysis is not a common feature of these diseases. Recurring episodes of ER are common in various breeds and with other ER-related disorders such as PSSM1 (below), but the term ‘recurrent exertional rhabdomyolysis’ (RER) is applied to a presumed inherited120 disorder of Thoroughbreds that are affected intermittently with episodes of ER, often without any clear inciting cause. Though possibly a result of an inherited trait from a common ancestral founder animal, it should not be assumed that a disorder with the same etiology is shared by all Thoroughbreds with intermittent bouts of ER, particularly given the potential acquired causes discussed above. Furthermore, it remains unclear whether this disease is unique to Thoroughbreds: certain similarities in vivo and in vitro suggest that other breeds, such as Standardbreds, and some Warmbloods, might share a similar, if not identical disorder.57,74,107 Although episodes of RER are associated with time away from training, and are themselves a welfare issue, intriguingly, ER-susceptible racing Standardbreds outperform matched controls, leading researchers to speculate that breeders have inadvertently selected for the RER trait57 as occurred in Quarter Horses with hyperkalaemic periodic paralysis (HYPP).121 There is in vitro evidence of underlying kinetic abnormalities of muscle in RER-susceptible Thoroughbreds: muscle from susceptible animals contracts more rapidly than normal muscle in vitro than does muscle from unaffected horses.107 Furthermore, authors have detected abnormal relaxation, though they disagree as to whether relaxation is hastened or slowed.107,122 The SR Ca2+- ATPase (SERCA) pump is a major determinant of muscle relaxation, but this pump’s activity is normal in extracted SR vesicles from horses with RER,123 suggesting that RER in Thoroughbred horses is not associated with abnormal SR Ca2+ uptake.
Muscle disorders of equine athletes
Introduction
General response of muscle to trauma and disease
Muscle damage caused by trauma, strains and tears
Recognition
History and presenting complaint
Physical examination
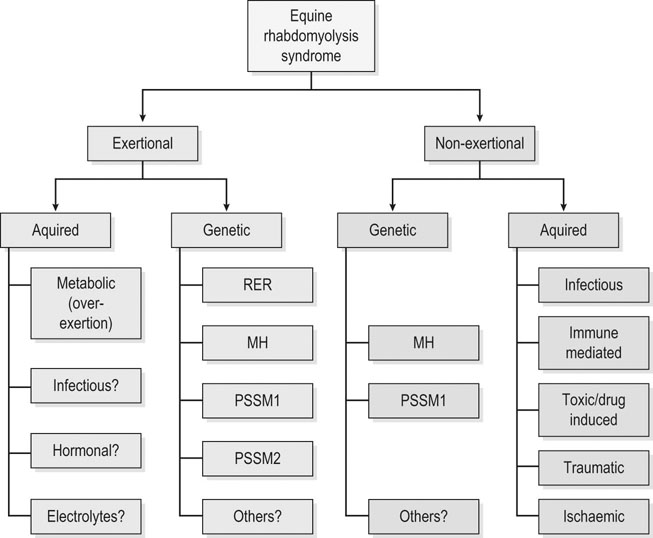
Exertional rhabdomyolysis syndrome
Recognition
History and presenting complaint
Physical examination
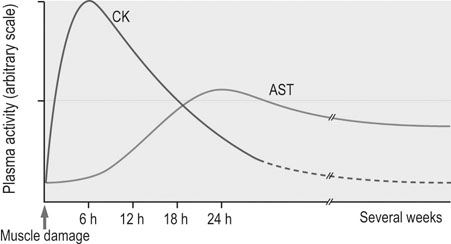
Laboratory examination
Blood sampling
Exercise testing
Urinalysis
Electrolyte clearance ratios

Muscle biopsy
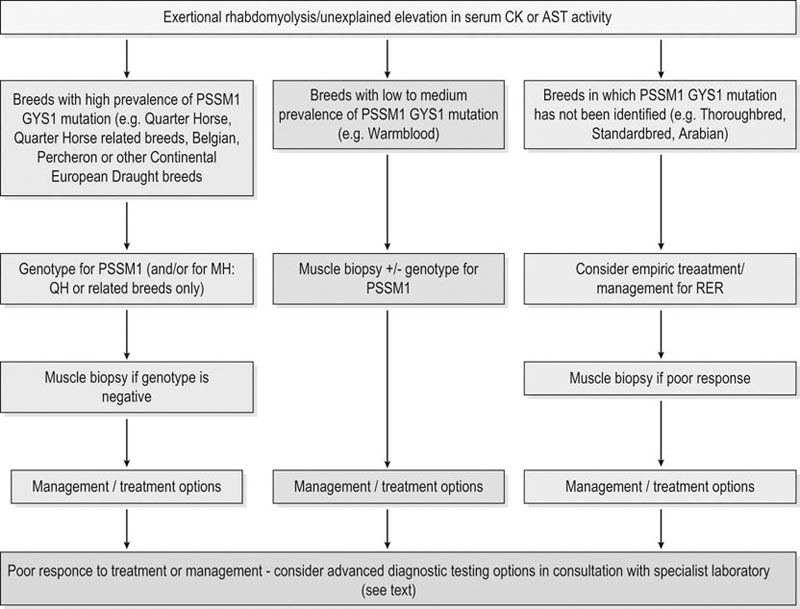
Genetic testing
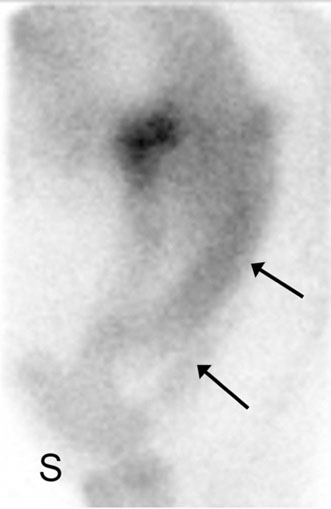
Scintigraphy
Additional tests
Necropsy examination
Diagnostic confirmation
Treatment and prognosis
Therapeutic aims
Therapy
Fluid therapy
Other therapies
Prognosis
Prevention
Diet
Electrolyte therapy
Dantrolene
Antioxidant supplementation
Etiology, pathophysiology and pathology
Acquired causes
Eccentric contraction
Metabolic exhaustion
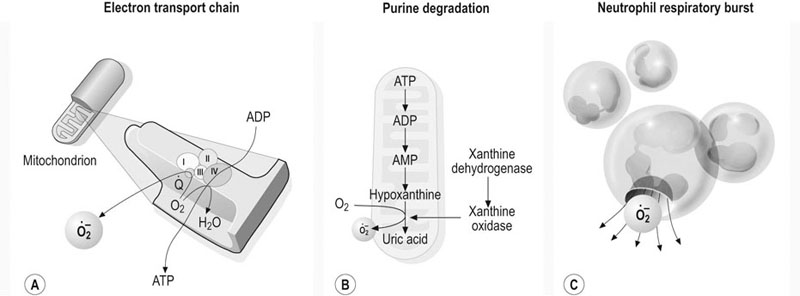
Oxidative injury
Electrolyte imbalance
Hormonal influence
Infectious causes
Inherited causes: recurrent exertional rhabdomyolysis
Etiology and pathogenesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Muscle disorders of equine athletes