Chapter 16 Monitoring Fluid Therapy and Complications of Fluid Therapy
Intravenous fluids are “drugs,” and fluid therapy is a “prescription,” and should be considered as such to avoid potential complications resulting from inappropriate selection, underdosing, and overdosing.59 Selection of fluid type and volume is a major component of the therapeutic plan and should include careful assessment of tissue and intravascular losses, acid-base and electrolyte status, age and species of the animal, nature of illness or injury, acute or chronic history, hematocrit and serum albumin concentration, coagulation status, cardiorespiratory function, and cost. The animal’s illness or injury is a dynamic event, and selection of fluid type and volume may change according to the patient’s response to fluid therapy and with improvement or deterioration of the underlying problem. Therefore constant monitoring to achieve desired endpoints is required. This chapter will introduce the various monitoring techniques frequently used in veterinary practice, the potential for misinterpretation, and complications associated with fluid therapy and catheters.
A thorough physical examination must accompany monitoring using the various technical devices available. Although monitoring central venous pressure (CVP), systemic arterial blood pressure (SABP), pulmonary capillary wedge pressure (PCWP), and cardiac output (CO) provides very useful (and sometimes essential) information, monitoring the patient by physical examination and biochemical evaluation of organ function also are very important, with improvement of these being the ultimate endpoints for achieving success with fluid therapy. As with the various monitoring devices, standard guidelines for assessing fluid deficits and overload by physical examination exist. However, there are many caveats, and interpretation is not necessarily clear-cut nor can it be assumed that absolute numbers or specific findings are related to fluid volume alone (Tables 16-1 through 16-3). Each patient must be assessed individually based on history, physical findings, and laboratory data.
Table 16-1 Physical Signs Associated with Dehydration
| Percent Dehydration | Physical Signs |
|---|---|
| <5 | Not detectable |
| 5-6 | Mild loss of skin elasticity |
| 6-8 | Definite loss of skin elasticity |
| May have dry mucous membranes | |
| May have depressed globes within orbits | |
| 8-10 | Persistent skin tent with slow return because of loss of skin elasticity |
| 10-12 | Persistent skin tent because of loss of skin Elasticity |
| Depressed globes within orbits | |
| Dry mucous membranes | |
| Signs of perfusion deficits (CRT >2 sec, tachycardia) | |
| 12-15 | Signs of shock |
| Death |
Note: The association between % dehydration and circulatory compromise must also be considered with rate of fluid loss. Chronic fluid loss may result in severe dehydration, but perfusion may be adequate; however, fluid loss occurring acutely will result in circulatory collapse at an estimated lower level of hydration. Therefore perfusion status cannot consistently be used to assess hydration status.
CRT, capillary refill time.
Table 16-2 Confounding Factors of Physical Findings Associated with Dehydration
| Assessment | Confounding Factors |
|---|---|
| Skin turgor (“tent”) | Young animals with subcutaneous fat |
| Obese animals with subcutaneous fat | |
| Cachectic animals | |
| Geriatric animals with loss of tissue elasticity | |
| Mucous membranes | |
| Dry | Panting, tachypnea, dyspnea |
| Moist | Nauseated, vomiting, drinking |
| Position of the globe | Cachexia |
| Perfusion status | Affected by rate of fluid loss; chronic loss may not affect perfusion parameters until a large volume is lost |
Table 16-3 Physical Findings Associated with Inadequate Tissue Perfusion
| Assessment | Confounding Factors |
|---|---|
| Mucous Membranes | |
| Pale pink | Vasoconstriction caused by pain or anxiety Anemia |
| Pale | Volume loss overestimated because of vasoconstriction caused by pain or anxiety |
| Dark pink or red | Vasodilatation and may be interpreted as normal volume |
| Hemoconcentration may be interpreted as normal volume | |
| Capillary Refill Time | |
| <1 sec may be considered adequate perfusion | |
| Difficult to interpret if peripherally vasoconstricted because of pain or anxiety |
Monitoring
Endpoints of fluid resuscitation
During administration of any fluid, basic monitoring techniques should be performed, including heart rate (HR), respiratory rate (RR), pulse pressure, capillary refill time (CRT), mucous membrane (MM) color, mentation, and temperature and color of the distal limbs and digits. Although abnormalities in these parameters may not be sensitive indicators of hypovolemia,60 a general goal for endpoints of resuscitation should be values within the normal range for the size and species of animal with recovery to normal mentation and warm, pink digits. Monitoring urine production with a goal of 0.5 to 1.0 mL/kg/hr also is a useful assessment of adequate volume, and 1.0 to 2.0 mL/kg is considered optimal with normal renal function, providing that the urine specific gravity is within a normal range of concentration (1.020 to 1.030). Lower urine specific gravity values with small volumes of urine may suggest a lack of concentrating ability, resulting in the urine produced rather than adequate volume and glomerular filtration rate. A higher urine specific gravity indicates the requirement for continuing fluid therapy in a patient with normal concentrating ability. When administering fluids to critically ill animals, measuring preload, stroke volume, or CO (targeted to specific values) is superior to measuring systemic blood pressure. Central venous and PCWP are surrogate markers of preload, but use of the pulmonary artery catheter (PAC) to measure the PCWP has been shown to be inconsistent, of questionable value, and associated with increased morbidity in human medicine and is being used less frequently.39 This technique is also difficult to perform in general practice and therefore not recommended. The CVP and SABP measurements frequently are used in veterinary medicine to guide fluid resuscitation. Measurement of CVP and SABP are associated with major pitfalls (see Arterial Blood Pressure and Central Venous Pressure section discussed later), but are still of value when used in conjunction with the physical examination. Suggested measurements for conditions requiring optimal resuscitation include 5 to 8 mm Hg (6.5 to 10.5 cm H2O) CVP, 80 to 100 mm Hg mean arterial pressure (MAP), and 100 to 120 mm Hg systolic blood pressure (SBP), and for patients in which the goal is adequate resuscitation (i.e., those with ongoing noncompressible hemorrhage), a MAP of 65 mm Hg and a SBP of 90 to 95 mm Hg are acceptable, physical findings are normal, until hemorrhage is controlled either spontaneously or surgically. Pulmonary contusions and other pulmonary conditions predisposing to capillary leak with increased hydrostatic pressure are additional indications for cautious adequate resuscitation. The patient’s base deficit or blood lactate concentration also may be used to assess perfusion. The goal should be to achieve an adjusted base excess of 0 to +4 mEq/L and a lactate concentration less than 1.4 mmol/L in cats and 2.0 mmol/L in dogs. Where a central line (jugular catheter) is in place, a recent study has identified venous saturation (ScvO2) of 68% in dogs to reflect a minimal adequacy of perfusion, which is similar to that reported in humans.31 However, as mortality significantly decreased above this value, it is recommended that fluid resuscitation with a goal of ScvO2 >70% be achieved.
Central venous pressure
The CVP is a measure of the hydrostatic pressure within the intrathoracic vena cavae.1,30 The CVP is slightly higher than the right atrial pressure (RAP), and RAP is quantitatively similar to right ventricular pressure at end diastole30 or preload.12 However, CVP does not reliably predict right ventricular end-diastolic volume.64 Accurate placement of the catheter and consistency in positioning of the animal are extremely important in interpretation of results and determination of trends.30 CVP measurements may be obtained from the caudal vena cava in cats.48 Keeping in mind potential pitfalls, measurement of the CVP during a fluid challenge, such as would be administered in hypovolemia or acute renal failure, can be valuable in assessing the effect of therapy. In the hypovolemic patient, for example, if no appreciable increase in CVP30 is observed after a fluid bolus, additional fluid or colloid should be administered (refer to Chapter 15 for an in-depth discussion of CVP ). It has been my experience that a rapid infusion of 20 mL/kg of a crystalloid may not be “tolerated” in some patients regardless of cardiovascular status (Table 16-4). Nausea, vomiting, shivering, and restlessness are frequently noted in these individuals (Box 16-1). The reason for the observed signs may be associated with a vagally mediated baroreceptor reflex secondary to atrial stretch. Should CVP increase above an acceptable range after such a challenge in an animal with acute renal failure, fluid administration should be curtailed or stopped (Table 16-4). These are general recommendations, and CVP does not reliably predict whether administration of a fluid bolus will or will not significantly increase CO under all conditions.66,67 Factors other than intravascular volume that influence CVP measurements include cardiac function (e.g., systolic or diastolic dysfunction), pulmonary hypertension (e.g., pulmonary thromboembolic disease), venous compliance (e.g., increased systemic vascular resistance), and intrathoracic pressure (e.g., pleural effusion, pneumothorax, pericardial effusion, mechanical ventilation). Although mechanical ventilation affects CVP, threshold values of CVP in ventilated patients still may be of value to predict hemodynamic instability when assessed in response to increasing airway pressure induced by positive end-expiratory pressure (PEEP).38 In this study of patients with acute lung injury, subjects with CVP less than 10 mm Hg usually had decreased CO when challenged with increasing PEEP, whereas those with CVP greater than 10 mm Hg had increased, decreased, or unchanged CO.
Table 16-4 Interpretation of CVP Values in Response to a Rapid Infusion of 20 mL/kg Crystalloid or 5 mL/kg Colloid20[30]
| Interpretation of Response | Response to Infusion |
|---|---|
| Euvolemia and normal cardiac function | 2-4 cm H2O increase from baseline returning to baseline in 15 min |
| Increased venous blood volume, reduced cardiac compliance, or both | An increase in CVP maintained >4 cm H2O above baseline |
| Normal blood volume | A slow (15 min) return to baseline |
| Increased blood volume relative to cardiac performance | A prolonged (>30 min) return to baseline |
| Markedly reduced intravascular volume; requires further resuscitation | Minimal to no increase in CVP |
| Reduced intravascular volume and accommodation of fluid within the intravascular space and subsequent reduction in vascular tone; requires further resuscitation | An increase in CVP with rapid (<5 min) return to baseline |
| Further resuscitation | Raise CVP by 2-4 cm H2O within first few minutes of bolus therapy. If falls rapidly to baseline, repeat bolus therapy until CVP 5-10 cm H2O (3-7 mm Hg) requiring 10-15 min to fall; at this point, blood volume and venous return are optimal relative to cardiac performance. |
| CVP ~7-9 cm H2O (10-12 mm Hg) with normal intrapleural and intraabdominal pressures | Higher volume may predispose to pulmonary edema; continued fluid resuscitation probably will not improve cardiac output |
Arterial blood pressure
Although systemic blood pressure is not an absolute measure of volume, it is frequently monitored during periods of bolus fluid administration when managing shock. When extensive monitoring is required, direct arterial pressure measurements should be obtained. However, on presentation, it may not be possible to successfully perform arterial catheterization, and pressures may be obtained with oscillometric or Doppler monitors. However, when the limbs are poorly perfused or the patient is cold, the oscillometric and Doppler methods are insensitive, and it is difficult to obtain accurate measurements, especially in small animals. In my experience, the coccygeal artery, with the cuff positioned as far proximal as possible, tends to be more reliable in this instance. The MAP is dependent on CO and systemic vascular resistance (SVR), according to the equation MAP = CO × SVR. Therefore adequate MAP does not necessarily indicate adequate CO if SVR is increased as may occur in a compensatory sympathetic response. During acute blood loss, especially in otherwise young healthy animals, the compensatory response can be quite dramatic and result in nearly normal or normal MAP. If resuscitation is based on normal MAP or SBP alone, inadequate resuscitation with continued poor perfusion likely will occur until the patient decompensates. However, if normal MAP or SBP is accompanied by a physical examination (see Physical Findings section) that indicates the presence of a sympathetic response, the clinician will be aware of the requirement for additional resuscitation or analgesics (Table 16-3). In this setting, it is difficult to know how much blood has been lost and the contribution of pain and anxiety. Pain, anxiety, and hypothermia also contribute to the sympathetic response, and the findings observed may be more a result of these factors than of fluid and blood loss. In this setting, intravascular volume loss may be overestimated, resulting in excessive fluid administration. Therefore fluid requirements and monitoring progress should be assessed based on several factors in addition to pressure measurements. These considerations include a relatively pain-free patient and an improvement in physical findings (see Physical Findings section).
Cardiac output measurements
Several techniques are available to measure CO, but most are technically challenging. The lithium dilution cardiac output (LiDCO) and PulseCO (both from LiDCO, London) have been investigated for use in humans,39 and in large45 and small animals.55 Briefly, isotonic lithium chloride is injected as a bolus via a central or peripheral vein, and a concentration-time curve is generated by an arterial ion-selective electrode attached to an arterial manometer system. The CO is calculated from the lithium dose and the area under the concentration time curve before recirculation. The PulseCO hemodynamic monitor was developed for use in conjunction with the LiDCO to give a beat-by-beat estimate of CO that is derived from analysis of the arterial trace. Although these systems have limitations, their use in veterinary research indicates potential value in clinical practice in anesthetized animals or nonmoving critically ill animals. Movement, flexion, and extension of the catheterized limb contribute to erroneous results (personal observations). A great advantage of this system is that a central catheter is not required, and continuous CO can be measured. Measuring CO during fluid resuscitation has definite advantages over determination of SABP because the former is a more accurate measure of volume. Predetermined goals for CO, stroke volume, and oxygen delivery can be set and monitored with this system. For critically ill patients being mechanically ventilated, intermittent cardiac output measurements can be obtained by the use of a partial CO2 rebreathing noninvasive system (NICO, Novametrix Medical Systems Inc, Wallingford, Conn.).15 This system requires body weight, Spo2, Fio2, Pao2, and Paco2, all of which are available in ventilated patients, and the CO2 sensor measurement obtained from expired CO2 at the endotracheal tube and ventilation circuit.21
Physical findings
As previously mentioned, SABP may be normal in patients with hypovolemia caused by blood loss, and therefore the physical examination must be considered in conjunction with SABP when assessing adequate resuscitation. Cool limbs, rectal temperature below normal, increased HR and RR, paler than normal MM color, prolonged CRT, and depressed mentation all indicate poor perfusion, regardless of blood pressure readings. If SABP is normal and the patient is free of pain but HR and RR are high and MMs still pale, a compensated stage of shock may exist, and further resuscitation is required. When assessing response to fluid therapy in animals with pain, an opioid analgesic (preferably hydromorphone or fentanyl) should be administered to control pain. The sympathetic response associated with pain and anxiety will be reduced, allowing the clinician to assess cardiovascular dynamics solely associated with the blood loss. Administration of these opioids commencing with a low-dose and careful titration to effect does not compromise the cardiovascular system47 and will allow better assessment of the patient because the effect of the sympathetic response to pain will be eliminated from consideration. It is advised, however, that depressed animals receive fluid therapy for a few minutes before opioid administration until mentation is improved. Depression (not associated with head trauma) indicates poor cerebral perfusion due to fluid or blood loss usually greater than 30% of the intravascular volume, and a potential slight reduction in SABP due to the opioid may compromise cerebral perfusion pressure further. Hypothermia may be a result of poor perfusion caused by low circulating volume or by a primary cause that may interfere with achieving resuscitation goals; clinical impression suggests this may be especially so in cats. Marked hypothermia results in bradycardia and decreased CO62; therefore, warming during resuscitation is necessary. Again, opioid analgesic administration should be administered once the patient’s mentation improves. Increased RR also may be associated with pulmonary injury, disease, or fluid overload. An improvement in attitude (i.e., improved cerebral perfusion) should be noted with adequate fluid resuscitation. The CRT and MM color, pulse pressure, and urine production also should improve. Palpation of the bladder and monitoring urine produced by assessing bladder size can be useful when urinary bladder catheterization cannot be performed.
Packed cell volume and total solids (or protein)
It is essential to obtain baseline packed cell volume (PCV) and total solids (TS) or total protein concentration on admission. During a traumatic event, sympathetic stimulation results in splenic contraction, especially in the dog, increasing the PCV and potentially giving the impression that hemorrhage has not occurred. A normal PCV may be observed after trauma even with clinically relevant blood loss. The TS in this setting will be lower than normal (6.0 to 8.0 g/dL), confirming blood loss. Monitoring these tests as frequently as every 15 minutes during resuscitation may be necessary to evaluate ongoing blood loss and the requirement for administration of red blood cells (PRBCs), whole blood, or hemoglobin-based oxygen-carrying solutions (HBOCs). A definitive recommendation regarding transfusion of blood products has not been established in veterinary patients, but the use of PRBCs, whole blood, or HBOCs is suggested when the PCV decreases to less than 25% in the dog or less than 20% in the cat, especially when ongoing resuscitation is required. The use of HBOCs may be an effective adjunct to limited resuscitation from hemorrhagic shock51 while reducing complications of aggressive fluid therapy, However, complications associated with these solutions have been reported in cats.20
Administration of a colloid has been recommended when the TS is less than 4.0 g/dL (less than 40 g/L) to avoid a clinically relevant decrease in colloid osmotic pressure (COP),50 which may predispose to tissue and pulmonary edema, especially when additional crystalloid fluids are to be administered. The refractometer reading for hetastarch is 4.5 g/dL (45 g/L) and that for pentastarch is 7.5 g/dL (75 g/L). After these colloids are administered, the TS measurement is difficult to interpret and cannot be extrapolated to a COP measurement. Response to administration of colloids must be assessed by direct COP measurement, CO determination, or improvement in clinical signs.
Urine production
During hypovolemia and dehydration, renal blood flow is decreased. When blood volume is decreased by hemorrhage, the decreased pressures result in activation of the sympathetic nervous system, including renal sympathetic nerves. Sodium and water are conserved by constriction of the glomerular arterioles, decreased glomerular filtration rate (GFR), increased tubular reabsorption of salt and water, and activation of the renin angiotensin aldosterone system. Decreased arterial pressure also results in secretion of antidiuretic hormone (ADH).28 Together, these actions serve to replenish the intravascular space and return blood volume toward normal. As a consequence of these effects, a very small volume of hypertonic urine is produced. In addition to renal blood flow and GFR, urine volume also is dependent on the concentrating ability of the kidneys. If underlying renal tubular dysfunction is present, increased urine volume may not reflect adequate renal perfusion and GFR. When renal function is otherwise normal, however, urine production and specific gravity are useful parameters to monitor when assessing intravascular volume. Urine output has been referred to as the “poor man’s cardiac output.” Intravenous fluid therapy also will expand the intravascular space and consequently increase urine volume.
Assessment of Urine Output
Careful monitoring is necessary to ensure that urine production is maintained by adequate fluid replacement (Chapter 22). Normal urine production is between 0.5 and 2 mL/ kg/hr but varies with the concentrating ability of the kidneys. The goal is to maintain urine output of 1 to 2 mL/kg/hr with a urine specific gravity of approximately 1.026 (dog) and 1.035 (cat). However, if there is loss of concentrating ability (e.g., renal tubular injury, Escherichia coli pyelonephritis), urine output can be extremely high (25 to 40 mL/kg/hr), and specific gravity may be in the hyposthenuric or isosthenuric range, hence the importance of measuring of urine output and specific gravity. If urine output is decreased, the patient should be assessed for possible third-space loss, capillary leak, increased temperature, vomiting, diarrhea, salivation, or inadequate resuscitation as causes. In addition, alterations in vasopressin (antidiuretic hormone or ADH) activity and intravascular sodium, may result in marked hyposthenuric polyuria or concentrated oliguria. [Chapter Hypernatremia, Hyponatremia]. Calculating appropriate fluid requirement is important because administering an excessive volume of fluids will result in diuresis, medullary washout, and electrolyte disturbances (especially potassium). Ongoing diuresis may require a prolonged hospital stay for correction of resulting fluid and electrolyte imbalances. Measurement of urine volume can be accomplished by:
1. Collection of urine when the animal voids
3. Intermittent or continuous urinary bladder catheterization
4. Placing preweighed towels or pads under the animal and weighing them after voiding. Any increase in towel or pad weight over baseline, unless otherwise soiled, is assumed to be the result of urine. The volume of urine voided can be estimated by assuming 1000 mL equals 1000 g (1 kg or 2.2 lb). This technique underestimates urine produced because some urine may remain in the cage.
1. Divide the day into six 4-hour intervals, four 6-hour intervals, or three 8-hour intervals.
2. Determine urine produced during each time interval, and add the estimated insensible and ongoing losses for that period.
3. Determine ongoing losses in vomitus, diarrhea, and saliva for the period selected.
4. Determine insensible loss at 20 mL/kg/day. In addition, for each degree Celsius above 38.5° C, add 10% of the normal daily maintenance fluid requirement (i.e., if the normal daily requirement is 1 L and temperature is 40.5° C, then 200 mL should be added). Divide this total amount by 6, 4, or 3 depending on the interval selected above.
5. This volume of fluid, in addition to the amount determined by urine produced and ongoing losses, is to be delivered during the next period. Daily weight is advised in all hospitalized patients because unnoticed polyuria or inadequate intravenous or oral fluids for an individual patient may result in weight loss, and excessive intravenous fluid administration may result in unnecessary weight gain.
Perfusion
Lactate
The blood lactate concentration may be used as an indicator of perfusion to monitor resuscitation and is discussed further in Chapter 10. Strict adherence to collection (i.e., blood should be collected in heparin) and processing (i.e., immediate) of blood samples is required. Lactate measurements can be performed on arterial or venous samples.34,43 Normal blood lactate concentrations are less than 2.0 mmol/L in dogs, with 3 to 5 mmol/L representing a mild increase, 5 to 8 mmol/L a moderate increase, and more than 8 mmol/L a severe increase. Normal blood lactate concentrations are less than 1.46 mmol/L in cats. When inadequate oxygen delivery to tissues occurs, cells revert to anaerobic metabolism, and lactate production increases. Obtaining an initial lactate measurement in all severely ill patients can serve as a useful method of evaluating severity of illness or injury. This was illustrated in a recent study of blunt trauma in dogs.74 Monitoring lactate concentrations as a method of measuring total body oxygen metabolism will provide information about the state of oxygen delivery and adequacy of resuscitation. With restoration of adequate perfusion and oxygen delivery, aerobic metabolism is resumed with a reduction in lactate production. A recent study in human septic patients demonstrated that lactate clearance derived from calculating the change in lactate concentration from two blood samples drawn at two time points (before and after treatment [e.g., fluid bolus]) was not inferior to ScvO2 measurements as a marker of adequacy of oxygen delivery. A lactate clearance of more than 10% ([lactate initial—lactate resuscitation/lactate initial]/100%) may be a useful monitoring technique in assessing improved perfusion status, especially in situations where appropriate equipment is not available to measure saturation of oxygen in central venous or jugular vein samples.40
The most common cause of hyperlactatemia is hypoperfusion and tissue hypoxia (type A), but increased lactate concentrations also may be caused by increased production secondary to alkalosis, hypoglycemia, various drugs, and systemic illness (type B).9 As an example, in those with acute liver failure and sepsis, hyperlactatemia is not necessarily associated with hypoxia. In this situation, the liver becomes a net producer of both lactate and pyruvate.8 Similarly, in those with sepsis, hyperlactatemia is a consequence of enhanced glycolysis and increased release of lactate from the intestine and the periphery. Therefore hypermetabolism must be considered as a cause for hyperlactatemia when no other indications of inadequate perfusion or tissue hypoxia are present.8 Type A hyperlactatemia also includes relative hypoxia, in which energy requirements exceed demand such as may occur in strenuous exercise and in extreme muscle activity (e.g., seizures, trembling, struggling).35 This effect resolves rapidly after cessation of the activity.
Acid-Base Status
Many illnesses and trauma are associated with acid-base disturbances, often, metabolic acidosis. Hypoperfusion and tissue hypoxia result in metabolic acidemia unless a comorbid condition results in metabolic alkalosis, generating a mixed disturbance in which blood pH may be within normal limits. In one study, 95% of animals referred to a tertiary referral center were diagnosed with metabolic acidosis.41 Knowing the metabolic status of a patient is an extremely important part of the overall assessment of the animal and provides information about the potential origin of the abnormality and the appropriate fluid to select. Eliminating the underlying problem ultimately will correct the abnormal metabolic status, but until it can be resolved, providing optimal therapy to improve outcome is essential. When blood gas analysis is not available, acidemic patients with 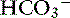 loss usually can be identified as having increased serum chloride concentration, decreased total CO2, and normal anion gap, information that can be obtained from a serum biochemistry profile. If acidemia is caused by the addition of an unmeasured anion (e.g., lactate, glycolate), the serum chloride concentration usually is normal, but the anion gap is increased. Where inappropriate administration of 0.9% sodium chloride is administered, a hyperchloremic metabolic acidosis frequently occurs. Alkalemic patients, however, often are hypochloremic. Monitoring acid-base status provides additional information about improved perfusion and resolution of the illness, as well as the potential need for a change in fluid therapy as the disease process changes. For example, a dog with vomiting caused by pyloric obstruction commonly will exhibit a hypochloremic metabolic alkalosis and hyponatremia; 0.9% sodium chloride is the fluid of choice. Once the underlying problem is resolved and alkalosis has been corrected, continuing with 0.9% sodium chloride may result in hyperchloremic acidosis; therefore a change to a balanced electrolyte solution typically is recommended.
loss usually can be identified as having increased serum chloride concentration, decreased total CO2, and normal anion gap, information that can be obtained from a serum biochemistry profile. If acidemia is caused by the addition of an unmeasured anion (e.g., lactate, glycolate), the serum chloride concentration usually is normal, but the anion gap is increased. Where inappropriate administration of 0.9% sodium chloride is administered, a hyperchloremic metabolic acidosis frequently occurs. Alkalemic patients, however, often are hypochloremic. Monitoring acid-base status provides additional information about improved perfusion and resolution of the illness, as well as the potential need for a change in fluid therapy as the disease process changes. For example, a dog with vomiting caused by pyloric obstruction commonly will exhibit a hypochloremic metabolic alkalosis and hyponatremia; 0.9% sodium chloride is the fluid of choice. Once the underlying problem is resolved and alkalosis has been corrected, continuing with 0.9% sodium chloride may result in hyperchloremic acidosis; therefore a change to a balanced electrolyte solution typically is recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


