Nicola Pusterla, Christian M. Leutenegger, Consulting Editors Christian M. Leutenegger • Nicola Pusterla Of the four classes of organic molecules that compose the basic physical structure of all living beings, nucleic acids are the only molecules that carry replicable instructive information. Nucleic acids differentiate themselves from lipids, carbohydrates, and even proteins in their ability to organize the basic unit of life, the cell. Even minor alterations in DNA and RNA molecules can disrupt the fine balance between health and disease. The field of molecular diagnostics seeks to elucidate the variations and mutations in genetic material that can cause disorder in the otherwise intricately organized body. The amplified era of molecular diagnostics for infectious diseases began in 1983 when Dr. Kary Mullis of Cetus Corporation first conceptualized the polymerase chain reaction (PCR). He went on to win the Nobel Prize in Chemistry for this revolutionizing technology 10 years later.1,2 Since the inception of PCR technology, infectious disease diagnostics has been at the forefront of molecular medicine, with the promise of detecting contagious pathogens in a safer and more sensitive way to aid in controlling their spread. Infectious disease testing is expected to continue to dominate the molecular diagnostic market for large animals in the foreseeable future. The use of amplified and nonamplified tests to assay the molecular makeup of the host rather than the pathogen is growing. Whereas cancer diagnostics is in its infancy for large animal testing, parentage and forensic testing together with single-nucleotide polymorphism (SNP) detection using in situ hybridization (ISH), fluorescent in situ hybridization (FISH), and sequencing technologies is gaining importance in assessing the presence and prognosis of genetic diseases. The ability of molecular diagnostic assays to sensitively and specifically detect the primary cause of a disease, with short turnaround time, is giving significant advantages to disease diagnostics. Molecular tests are able to detect a specific unknown. Such tests are binary by nature; they provide a yes-or-no answer. However, certain tests when used quantitatively provide more insight into the medical manifestation induced by Clostridium perfringens toxins, for example. The clinically valuable information rests in determining the presence or absence of a specific pathogen in a biological sample derived from an animal. Because detection of viral, bacterial, rickettsial, fungal, or parasitic nuclear material in a biological sample gives clinically important information, molecular tests have direct clinical utility. However, the clinical context is always important to consider when making an interpretation on a molecular test. Determining the presence of a gene sequence per se without the clinical context does not necessarily correlate with a given clinical action, since diseases are generally multifactorial. In contrast, establishing the presence of the neuropathogenic form of equine herpesvirus (EHV)-1 in an equine patient’s blood sample has direct association with EHV-1 infection, whether this translates into a symptomatic stage or not. The binary nature of molecular tests, which establishes the presence or absence of genetic material in a sample with a high degree of accuracy, has an accepted clinical value for infectious disease diagnostics because of its correlation with clinical signs. Rapid results have tremendous clinical value in curtailing infection. Molecular methods can provide results in hours compared with days for culture-based or alternative technologies. The high sensitivity and specificity of molecular testing is particularly valuable in early determination of infection and makes it advantageous compared with antibody and antigen testing without amplification techniques. In most cases pathogens enter the host and replicate exponentially; early detection provided by the higher sensitivity allows for earlier clinical intervention and a better prognostic outlook. Molecular tests have been shown to produce fewer false-positive and false-negative results compared with other test platforms. Therefore, molecular tests are becoming the gold standard in the laboratory in terms of sensitivity and specificity. Key features for the adoption of molecular diagnostics for infectious agents are as follows: • Superior sensitivity and specificity compared with most immunoassays • Automated platforms that significantly increase throughput • Quantitative assessment of viral load, which is clinically useful • Fast turnaround time that speeds detection and reduces overall costs • Simultaneous analysis of multiple analytes • Standardized environment with the potential for full comparability across lab systems The relative technological superiority of molecular methods makes them likely to partially replace other types of more conventional methods such as culture-based tests or certain direct antigen and antibody tests to determine the presence of an infectious pathogen in a sick animal. Whereas antibody testing is more of a broad screening tool, molecular diagnostics allow accurate detection of the genome of a pathogen in a clinically sick animal. Culture-based tests have a turnaround time of days and are labor intensive. In addition, certain infectious agents are difficult to culture or biohazardous for laboratory personnel (e.g., Mycobacteria species, fungi, Mycoplasma species, Lawsonia intracellularis). In such instances, molecular detection of selected pathogens has already supplanted conventional culture. Rapid and specific detection of infectious disease agents is crucial for the prevention or containment of outbreaks. In the case of avian flu, the rapid detection of H5N1 strains of that virus is critical for containment. The avian flu pandemic concerns necessitate rapid and high-throughput molecular tests to maintain the well-being of animal and human community health. Similarly, West Nile virus (WNV) screening of mosquito pools and birds as well as screening for exotic Newcastle disease are additional examples of high-throughput applications with predominance for molecular tests. Since the advent of molecular platforms in the diagnostic laboratory, most molecular tests in veterinary medicine are performed by several commercial and public laboratories (Table 29-1). Unlike the market in human molecular diagnostics, which is dominated by four large players (Roche, Bayer, Gen-Probe, and Abbott), veterinary molecular diagnostics shows significant fragmentation in particular with many university diagnostic laboratories. However, the transition to the more advanced real-time PCR platform within the past 5 to 7 years and the abandonment of conventional PCR tests by many laboratories has led somewhat to a concentration process in the veterinary diagnostic landscape for a good reason: real-time PCR is the more advanced method and is considered a fully matured technological platform with great incentives for automation, operational efficiency, and affordability. TABLE 29-1 Examples of Molecular Diagnostic Laboratories in the United States* * American Association of Veterinary Laboratory Diagnosticians (AAVLD)-accredited laboratories are listed if they offer commercial molecular diagnostic services available to veterinarians. Parallel testing of multiple infectious agents in highly standardized platforms is a central advantage of molecular assays; it essentially allows several tests for both DNA and RNA pathogen targets to be performed simultaneously on a single sample, therefore condensing the medical message obtained on a given diagnostic sample. This development is a noteworthy driver for molecular diagnostics because it allows acquisition of more meaningful data from a single sample. This so-called panel strategy allows an efficient workup of complex clinical syndromes with general symptomatology that do not allow etiologic diagnosis based on clinical signs. These clinical cases are complex for the decision-making process for the veterinarian dealing with patients unable to communicate the pain matrix. In complex organ-related problems with general or unspecific symptomatology, multiple infectious agents can be responsible for a clinical picture or aggravate other primary events in the differential workup. Even though veterinarians tend to make a single-pathogen diagnosis, it has become more evident in recent years that many syndromes are caused by multiple infections present at a given time. Panel testing on a large scale will uncover unknown co-infections in animals, which can diffuse the clinical picture. It has long been speculated, for example, that seemingly clinically irrelevant EHV-2 infections in horses may be a predisposing factor for Rhodococcus equi pneumonia in foals. More characteristic examples include respiratory infections in companion animals, often initiated by subclinical viral or bacterial infections that lead the way to secondary infections. In addition, many vectorborne pathogens have a high tendency to persist in infected animals and therefore may facilitate viral infections or aggravate preexisting conditions such as feline immunodeficiency virus or feline leukemia virus infection. Sepsis caused by infectious agents is expected to become an important segment for equine molecular diagnostics, especially in neonatal medicine. Molecular diagnostics enable fast turnaround time with rapid initiation of treatment. In the future, panel testing for the most important sepsis-inducing agents could be complemented by the addition of assays targeting antimicrobial resistance genes. This would allow the modification of treatment regimens in case antimicrobial resistance is detected. Diagnostic tests such as PCR assays offer the potential for fast and accurate determination of the presence of an infectious agent, which can lead to an improved clinical outcome because of the faster initiation of more etiologic-based treatment and the possibility for quantitative treatment monitoring. Even if molecular-based tests are more expensive than more traditional diagnostic assays, their overall impact will lead to reduction in treatment costs. Key indications for use of a molecular test are the speed and accuracy of molecular assays. In comparison with traditional culture for bacteria, rickettsial organisms, fungi, and viruses, molecular testing offers direct detection of the target pathogen within a fraction of the time. Owing to its speed, PCR assays have the potential to replace traditional culture methods for infectious agents that are difficult to culture or not cultivable at all. Compared with serology testing, molecular tests offer the advantage of detecting an infectious agent before an immune response occurs and a detectable antibody titer is developed. Immunoglobulin M (IgM) analysis in certain applications (such as WNV) can alleviate this problem; however, molecular testing methods are more reliable in picking up early virus replication and allow a faster epidemiologic assessment and earlier patient treatment. In certain instances, for the discrimination of a clinically relevant EHV-4 infection from a latent infection, the detection of viral DNA is not informative. In such cases the quantitative assessment of viral loads is necessary.3 Similarly, the choice of RNA or DNA affects the ability to distinguish disease (active virus replication and production of viral transcribed RNA) from nondisease (viral DNA of latently infected cells) status. The main methodologies used for molecular diagnostics include the following: • Nucleic acid capture, probe hybridization • Isothermal amplification of nucleic acids4 • Transcription-based amplification methods • Signal amplification by branched-chain DNA • Qualitative pathogen identification using nucleic acid amplification Genotyping assays are usually used to test for known mutations associated with inherited genetic conditions such as hyperkalemic periodic paralysis (HYPP) in horses, an autosomal dominant condition that causes potassium-induced attacks of skeletal muscle paralysis. Many of these assays depend on sequence-specific probes designed to hybridize with known genetic variations. For infectious agents, genotyping or speciation is achieved by using highly specific PCR- or hybridization-based assays or by restriction enzyme fragment length polymorphism (RFLP) assays. In these assays, amplified material is digested with a certain restriction enzyme that characterizes the difference between two target sequences. Because of its predominance in research and diagnostic applications, PCR assays will be discussed in more detail in later chapters. To give practitioners guidance about what to look for when PCR-based molecular diagnostic assays are offered, we will discuss some aspects such as design guidelines, differences between traditional and real-time PCR assays, sampling, controls, and interpretation of results in greater depth. PCR testing in its pure form is a three-cycle event: denaturation of double-stranded target (or in later cycles PCR products), annealing of target and primers, and extension of the DNA strand from the primer. Real-time PCR testing was introduced into the marketplace in 1996 and replaced the tedious gel electrophoresis step to detect PCR products after amplification. In one survey, 98% of equine veterinarians knew about PCR testing and 79% of equine veterinarians at universities knew the difference between conventional and real-time PCR testing.5 During gel electrophoresis, the PCR products are separated by size and visualized using a dye (ethidium bromide) that intercalates with the double-stranded DNA. Real-time PCR detects the PCR products by using an internal probe that is labeled with two fluorescent dyes: a reporter dye and a quencher dye. The fluorescent activity of the reporter dye is absorbed (quenched) by the quencher dye. The probe binds to the PCR products between the two PCR primers. If the primers are extended by the DNA polymerase, the 5′ nuclease activity of the DNA polymerase digests the internal probe (hence hydrolysis probe) and releases the quenched fluorescence. Alternatively, hybridization probes can be used that are not digested during amplification and that allow melting curve analysis. This single change in the protocol regarding how to detect the PCR products revolutionized the use of PCR testing for both research and diagnostic applications. Most of the advantages resulting from this principle originate from the fact that the PCR tube does not have to be opened after a PCR assay for analysis. This principle is called closed-tube detection. The advantages are as follows: 1. Because of the closed-tube detection format, PCR products cannot escape from the PCR reaction containers. If escape occurs, it leads to the contamination of the PCR laboratory and subsequently the next PCR reactions. The consequence is false-positive PCR results. Real-time PCR efficiently eliminates this risk of PCR product carryover. There is also a second safety system called AmpErase UNG (Invitrogen, Grand Island, NY),6 which eliminates contaminating PCR products. In general, molecular diagnostic laboratories provide strict recommendations for sample collection, including shipping instructions. These instructions include specimen type, volume, anticoagulant, and specimen transport, storage, and handling. The sample type is largely influenced by the pathogenesis of the disease and plays a key role in the performance and interpretation of the test results. Veterinarians are advised to adhere to these recommendations, because the quality of the result is directly correlated to the quality of the sample and preservation of the nucleic acid content. Molecular assays often offer the convenience of using a small specimen acquired with a minimally invasive procedure. The diagnosis of herpesviruses is a classic example in which culture and neutralization assays have been largely replaced by PCR testing on a small volume of aspirate or swabs from mucosal surfaces. Molecular tests can detect the presence of small numbers of organisms, and the probability of detection increases when a larger volume of specimen is added to the amplification reaction. Because molecular assays do not need viable organisms for testing, more flexibility in specimen transport is possible than with culture methods. Appropriate specimen collection and transport conditions are important to ensure successful extraction of intact nucleic acid and to prevent cross-contamination. Specimen transport and storage conditions are likely to vary among specimen types and between RNA and DNA tests. RNA is more susceptible to degradation but in general, molecular diagnostic samples should be cooled in order to slow the nucleic acid degradation within the sample. Because of the difficulty of maintaining a freeze chain, molecular diagnostic samples should only be cooled throughout the collection, storage, and transportation process in order to minimize sample deterioration. Detailed storage and shipping instructions are crucial for a successful molecular diagnostic workup. Practitioners should be aware of these recommendations and consider using appropriate cooling containers when samples are collected in the field. Such samples maintain stability if stored appropriately in a cooled environment. Freezing and in particular the thawing process often adversely affects the quality and should be avoided if not otherwise instructed by the laboratory. Sampling errors are among the many preanalytic variables that can affect the outcome of the results. It is therefore recommended to have the appropriate labeling material available for blood containers or other sample types. Blood or body liquid contamination on the outside of the containers is an obvious cause of sample cross-contamination before the samples are manipulated in the laboratory. Proper collection procedures are essential to prevent these kinds of artifacts. Many commercially available manual and automated methods have been successfully applied to infectious disease testing using a variety of clinical materials. Especially in veterinary molecular diagnostics, the variety of sample types can be challenging for processing through a single platform but can be achieved by validating sample type specific pretreatment procedures in order to create a lysate of similar viscosity and nucleic acid concentration, which maximizes the extraction efficiency. Automated or semiautomated platforms are rapid and usually require only a small volume of specimen. In general, these systems are total nucleic acid extraction systems and support the parallel analysis of DNA and RNA pathogens from the same sample in a panel configuration. In addition, commercially available automated systems can reduce hands-on labor requirements and speed up the process significantly, leading to even shorter turnaround times. Most systems can accommodate sample volumes ranging from 100 to 500 µL and even 1 mL of specimen lysate. Nucleic acid binding capacity varies and can be as high as 200 µg per individual extraction position, which allows the maintenance of a good limit of detection even when many infectious agent targets are analyzed in parallel for a given panel. Interpretation of results obtained with molecular assays for infectious diseases requires understanding of the pathogenesis and biology of the target organisms. Some challenges are unique to molecular tests and are different from considerations in interpreting other microbiologic tests. Such differences are related to the distinction of viable from nonviable organisms and the correlation of nucleic acid detection with presence of disease or disease association. Interpretation of a negative result includes information about the sensitivity and nucleic acid extraction efficiency. A false-negative result may be caused by a degraded or unstable sample. Insufficient volume or inappropriate sample type, inadequate sampling procedures, and transport problems are additional sources for potentially false-negative results. Sample-specific internal positive controls targeting endogenous genes such as the universal 18S rRNA (ssrRNA) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene help to identify these problems. These so-called internal positive controls allow the quantification of the sample specific nucleic acid (both DNA and RNA) and allow validation of a diagnostic sample for its use in PCR; samples below a certain quantity of DNA are at risk of being called false negative; therefore, repeat collection can be recommended. In addition, inhibition phenomena originating from difficult sample matrixes such as feces, urine, or environmental samples contaminated with trace amounts of soil (humic acid) have to be controlled with internal positive controls to assess the inhibitory effects. The factors requiring consideration for the interpretation of positive results include assay specificity and contamination issues. PCR testing or any other target amplification method is subject to these considerations. Real-time PCR testing using closed-tube detection procedures reduces the risk of PCR product carryover as a source of false-positive results and the availability of systems, which mark the PCR products when produced with certain types of nucleotides (uracil instead of thymidine), allows the specific degradation of contaminating PCR products before the next PCR amplification is taking place (AmpErase UNG system). Combined with air movement control in the molecular diagnostics lab (overpressurized clean lab and negative-pressurized sample preparation lab) and limited access to trained laboratory technicians, great safety can be achieved, leading to valid results. In general, molecular assays do not provide information about the viability of an infectious agent. Exceptions to this are DNA viruses, bacteria, and parasites analyzed for transcribed genes instead of their genomic DNA. This approach allows the assessment of the replication competence of a target and therefore is highly correlated with viability. Examples are toxin quantification, differentiation of vaccination versus infection, and herpesvirus quantification in order to differentiate between lytic (acute) and chronic (latent) infection, which has immediate implications to the ability of the virus to cause or contribute to clinical signs. In addition, spliced RNA occurs only if viral genes are actively transcribed and provides a means for obtaining information about the replication activity of a virus and the stage of infection. In other cases, targeting the ribosomal RNA of parasites such as Toxoplasma and Cryptosporidium is a means for obtaining viability information and also may increase the analytic sensitivity.7 Detection of the nucleic acid of a pathogen does not necessarily ensure that the organism is the cause of the disease. A primary example is herpesvirus infections (EHV-1 and EHV-4), in which the detection of DNA may indicate the presence of lytic, non-replicating, or latent virus. Studies indicated that high viral loads of EHV-4 DNA allow the formulation of a cutoff value to differentiate between lytic and latent infection.8 In this particular case, high viral loads were associated with the presence of clinical signs and the presence of viral RNA transcripts. Therefore, quantitative real-time PCR testing can provide a means to obtain much improved disease association and invalidates the notion that PCR “just detects dead DNA.” Reporting results for qualitative assays in infectious disease monitoring is simple: a sample either does or does not contain the nucleic acid of a target organism. Further relevant information includes the nucleic acid extraction efficiency, nucleic acid stability, and sample integrity determined with the various quality controls. Reporting results for quantitative molecular infections is more complex. For veterinary medicine, some quantitative applications have been established and are progressively phased into diagnostic routine analysis. Such quantitative PCR tests combined with fully standardized protocols will continue to expand within the veterinary diagnostic market as our understanding of the biology and pathology of infectious agents grows. Veterinary molecular diagnostics is an emerging market with a growing level of regulation. Standards such as those defined by the American Association of Veterinary Laboratory Diagnosticians (AAVLD; http://www.aavld.org), Molecular Diagnostic Methods for Infectious Diseases (NCCLS; www.nccls.org), or MIQE (minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments) are adopted by commercial laboratories aiming to provide a comprehensive diagnostic service. Other sources for guidelines are U.S. Food and Drug Administration (FDA) guidelines (www.fda.gov) for the detection of nucleic acids; ASTM guidelines (Standard Guide for Detection of Nucleic Acid Sequences by the Polymerase Chain Reaction Technique; www.astm.org); and the Association for Molecular Pathology (AMP) (Recommendations for In-House Development and Operation of Molecular Diagnostic Tests; www.ampweb.org). Veterinarians can use a variety of guidelines to select laboratories for molecular diagnostics. However, there are some major factors worth considering before samples are submitted. First, it is worthwhile to obtain information about the nature of the PCR test (traditional vs. real-time). Second, questions addressing the quality control and quality assurance system within a particular laboratory should be asked. Third, turnaround time, pricing, and the level of guidance with result interpretation and availability of consulting resources are additional factors worth investigating before samples are submitted. As with any methodologies, quantitative PCR is a work in continuous progress and development and improvement. Although all of the methods described in this review use the Nobel Prize–awarded PCR process, the advantages and disadvantages of subtle differences in assay format, accuracy, and reliability of quantitation have to be rigorously compared. The next generation of quantitative real-time PCR processes will be increasingly automated, standardized, and miniaturized. The time for nucleic acid preparation and PCR amplification will be further reduced by using small microtiter-formatted microfluidic cards or silicon-based chip technology.9–11 Amplification time has been brought down to several minutes by using an advanced nucleic acid analyzer (ANAA) consisting of a battery-powered array of silicon-based PCR microchips with thin-filmed resistive heaters, thus enabling ultrafast amplification, further pushing the technological possibilities in order to improve the diagnostic results output.10 How will these new systems be incorporated in routine veterinary diagnostics? Veterinary medicine will experience a broad adoption of many quantitative assays for infectious agents and genetic abnormalities in the near future. The ability to standardize assays will allow high-throughput applications that have not been possible with traditional molecular methods. For this reason, applications for veterinary medicine may experience not only quantitative but also qualitative growth. These assays will help improve patient management and client satisfaction. Nicola Pusterla • Christian M. Leutenegger The ready availability of a correct etiologic diagnosis, particularly in contagious infections, enables the veterinarian to make early decisions regarding the patient’s care and management, address appropriate treatment, and allow timely notification and discussion of management issues pertaining to the prevention of disease spread. The past two decades have seen a revolution in the understanding, management, diagnosis, control, and prevention of infectious diseases.1,2 This period has encompassed the discovery of emerging equine agents, antimicrobials and vaccines, as well as a wealth of improved diagnostic tests for equine practitioners. Despite these advances, infectious diseases remain a leading cause of equine morbidity and mortality, with resurgence of certain infections (e.g., West Nile virus), an increasing population of elderly, more susceptible horses, and an increasing international equine commerce, expanding the geographic distribution of pathogens.3,4 The focus of rapid diagnosis of infectious diseases has also shifted during this time. The most obvious change has been the appearance and increasing importance of nucleic acid (NA) amplification-based techniques, primarily the polymerase chain reaction (PCR), at the expense of traditional methods of clinical microbiology.5 The PCR has become an increasingly important tool in microbial diagnosis in recent years, mainly because of its rapidity, high sensitivity, and high specificity. These superior characteristics have propelled the field of PCR-based molecular diagnostics into the arena of applied diagnostics for infectious agents. Because the number of published and offered PCR assays is steadily rising, there is a need for critical evaluation, comparison of performance, and eventually standardization of methods to enable equine practitioners to select the optimal methodology. Nucleic acid (NA) techniques to detect the presence of infectious agents in biological specimens require stringent quality guidelines. These guidelines aim to ensure the stability of NAs (both genomic DNA and total RNA), which are the target for molecular-based diagnostic methods. Whole blood samples are collected aseptically into evacuated blood tubes containing EDTA; body fluids (e.g., thoracic, abdominal, joint, cerebrospinal, tracheal wash, bronchoalveolar, and guttural pouch lavage fluid) and tissues should be collected into serum tubes without additives; nasal or nasopharyngeal secretions should be collected with rayon- or Dacron-tipped swabs and are best kept in a sterile serum or conical tube (virus transport medium is recommended for the detection of viruses); fecal material should be collected into small fecal cups or serum tubes (Table 29-2; Fig. 29-1). All samples must be sent cooled on blue ice by express mail overnight to the laboratory. Freezing of samples should be avoided. Short-term storage for a period of 2 to 3 days before shipment (over a weekend) should be done in a refrigerated compartment. Each sample should be properly labeled and accompanied by a submission form containing information pertaining to the patient, owner, veterinarian, sample, and pathogen (s) to be tested (most submission forms can be downloaded from the respective laboratory website). The laboratory should be notified in advance and inquiry should be made about the availability of the offered tests as well as turnaround time and associated costs. Incoming samples normally are processed the same day and PCR results usually are available within 24 to 48 hours if the NA passes quality control. TABLE 29-2 Tissue Samples Commonly Used for the Molecular Detection of Common Equine Pathogens BAL, Bronchoalveolar lavage fluid; CNS, cerebrospinal tissue; CSF, cerebrospinal fluid; GPL, guttural pouch lavage; NPL, nasopharyngeal lavage; NPS, nasal/nasopharyngeal swab; TW, tracheal wash fluid. An array of NA amplification techniques may be offered by laboratories in order to establish an etiologic diagnosis of equine infectious diseases. Several published studies have shown the benefit of NA amplification techniques in comparison to conventional microbiological techniques. Current efforts are aimed at improvement of the diagnostic efficiency of molecular techniques, both for common and less common infections. To facilitate a decision on which pathogens should be evaluated for a specific case, some laboratories offer panels covering specific organ systems (e.g., respiratory, gastrointestinal, neurology, bloodborne). Such panels test several common pathogens for each organ system. Some of the diagnostic PCR applications most relevant for equine practice are presented as follows, along with their advantages and potential pitfalls. Respiratory pathogens are often contagious, and infections must be diagnosed rapidly in order to prevent a disease outbreak and institute the appropriate management plan. The short turnaround time and reliability of PCR makes this molecular technology an ideal tool for the diagnosis of respiratory pathogens. Equine influenza is commonly diagnosed by virus isolation or detection from nasopharyngeal swabs collected from horses during the early febrile stage of the disease. Virus can be grown from nasopharyngeal swabs by inoculation and incubation of embryonated chicken eggs or passage through Madin-Darby canine kidney cells. Although isolation of the virus is essential to allow antigenic and genetic characterization of the strain, this technology is time consuming and successful isolation is, at best, to be expected in 50% of the cases. Further, since mutations have been reported to occur during the process of viral culture, recovered isolates must be viewed critically. In recent years new methods for virus detection, such as antigen-detection enzyme-linked immunosorbent assays (ELISAs) and PCR, have been described. Collectively these detection methods have shown higher sensitivity than virus isolation and antigen-capture ELISAs (Table 29-3).6–9 Amplification of the single-stranded RNA of equine influenza viruses is performed by reverse transcription-PCR (RT-PCR) technology, using either a one-step, nested, or real-time approach. The hemagglutinin, nucleoprotein, and matrix gene are the commonly targeted genes for these molecular assays. Unfortunately, comparison of the different PCR assays is precluded by the use of different technologies, the lack of standardization among the assays, and variation in targeted genes. Nucleotide and deduced amino acid sequences of portions of the hemagglutinin gene are now routinely used for phylogenetic characterization of outbreak strains. Further, novel real-time assays can be used as a viable replacement for the more traditional methods of quantifying equine influenza virus in vaccine efficacy studies. Another advantage of assays such as antigen-detection ELISAs and PCR is their ability to detect nonviable virus, a situation that may occur when nasopharyngeal samples are frozen or not adequately stored and/or shipped to a diagnostic laboratory, allowing a rapid diagnosis in the acute phase of infection. The commercially available Directigen Flu A (Becton, Dickinson and Company, Franklin Lakes, NJ) detects nucleoprotein, one of the type-specific antigens of type A influenza viruses, and was designed for use in cases of human influenza infection. Although this assay has the shortest turnaround time of all antigen tests (i.e., 15 minutes), its sensitivity decreases when low titers of virus are present, for example, in a partially immune horse with subclinical signs or in a clinical case past the acute onset of disease. In recent years, PCR-based assays have been described for the identification of equine influenza. TABLE 29-3 Comparison of Different Viral Detection Methods Using Nasopharyngeal Secretions from Naturally and Experimentally Infected Horses with Equine Influenza Virus
Molecular Diagnostics in Large Animals

Molecular Diagnostics in Large Animals
Technological Superiority of Molecular Tests
Rapid and High-Throughput Applications Promote Molecular Tests
Increasing Adoption of Molecular Tests by University and Commercial Laboratories
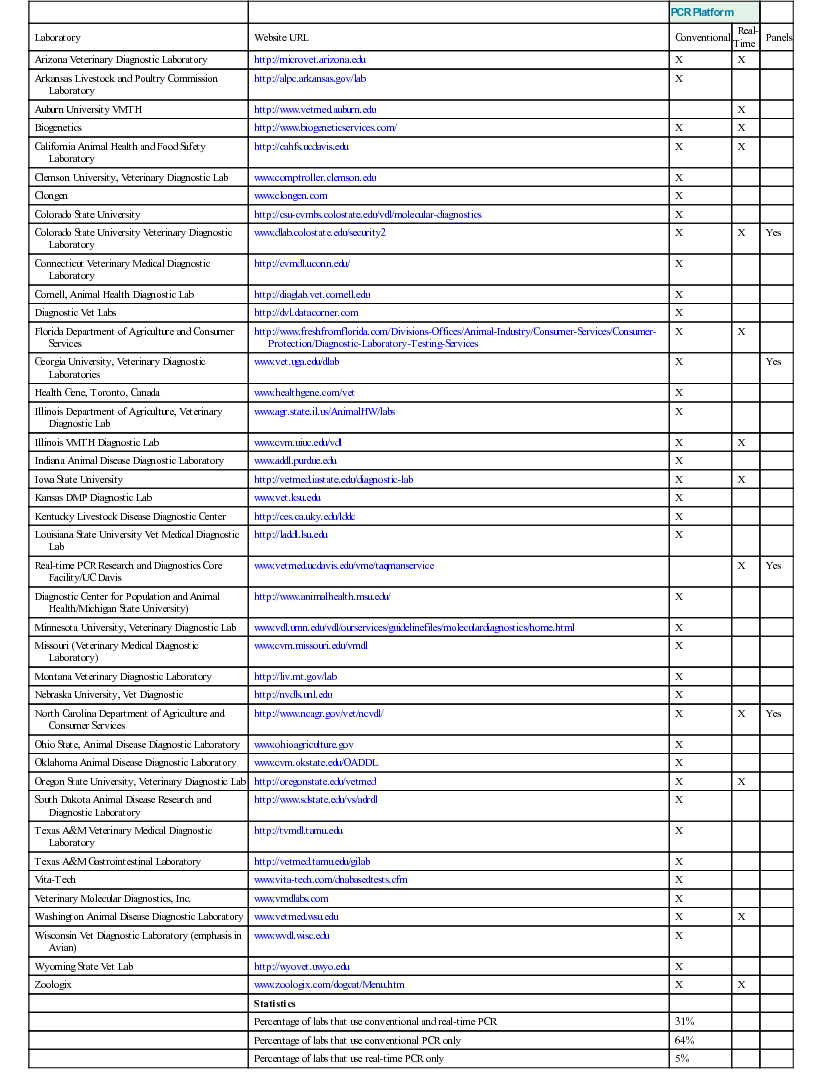
PCR Platform
Laboratory
Website URL
Conventional
Real-Time
Panels
Arizona Veterinary Diagnostic Laboratory
http://microvet.arizona.edu
X
X
Arkansas Livestock and Poultry Commission Laboratory
http://alpc.arkansas.gov/lab
X
Auburn University VMTH
http://www.vetmed.auburn.edu
X
Biogenetics
http://www.biogeneticservices.com/
X
X
California Animal Health and Food Safety Laboratory
http://cahfs.ucdavis.edu
X
X
Clemson University, Veterinary Diagnostic Lab
www.comptroller.clemson.edu
X
Clongen
www.clongen.com
X
Colorado State University
http://csu-cvmbs.colostate.edu/vdl/molecular-diagnostics
X
Colorado State University Veterinary Diagnostic Laboratory
www.dlab.colostate.edu/security2
X
X
Yes
Connecticut Veterinary Medical Diagnostic Laboratory
http://cvmdl.uconn.edu/
X
Cornell, Animal Health Diagnostic Lab
http://diaglab.vet.cornell.edu
X
Diagnostic Vet Labs
http://dvl.datacorner.com
X
Florida Department of Agriculture and Consumer Services
http://www.freshfromflorida.com/Divisions-Offices/Animal-Industry/Consumer-Services/Consumer-Protection/Diagnostic-Laboratory-Testing-Services
X
X
Georgia University, Veterinary Diagnostic Laboratories
www.vet.uga.edu/dlab
X
Yes
Health Gene, Toronto, Canada
www.healthgene.com/vet
X
Illinois Department of Agriculture, Veterinary Diagnostic Lab
www.agr.state.il.us/AnimalHW/labs
X
Illinois VMTH Diagnostic Lab
www.cvm.uiuc.edu/vdl
X
X
Indiana Animal Disease Diagnostic Laboratory
www.addl.purdue.edu
X
Iowa State University
http://vetmed.iastate.edu/diagnostic-lab
X
X
Kansas DMP Diagnostic Lab
www.vet.ksu.edu
X
Kentucky Livestock Disease Diagnostic Center
http://ces.ca.uky.edu/lddc
X
Louisiana State University Vet Medical Diagnostic Lab
http://laddl.lsu.edu
X
Real-time PCR Research and Diagnostics Core Facility/UC Davis
www.vetmed.ucdavis.edu/vme/taqmanservice
X
Yes
Diagnostic Center for Population and Animal Health/Michigan State University)
http://www.animalhealth.msu.edu/
X
Minnesota University, Veterinary Diagnostic Lab
www.vdl.umn.edu/vdl/ourservices/guidelinefiles/moleculardiagnostics/home.html
X
Missouri (Veterinary Medical Diagnostic Laboratory)
www.cvm.missouri.edu/vmdl
X
Montana Veterinary Diagnostic Laboratory
http://liv.mt.gov/lab
X
Nebraska University, Vet Diagnostic
http://nvdls.unl.edu
X
North Carolina Department of Agriculture and Consumer Services
http://www.ncagr.gov/vet/ncvdl/
X
X
Yes
Ohio State, Animal Disease Diagnostic Laboratory
www.ohioagriculture.gov
X
Oklahoma Animal Disease Diagnostic Laboratory
www.cvm.okstate.edu/OADDL
X
Oregon State University, Veterinary Diagnostic Lab
http://oregonstate.edu/vetmed
X
X
South Dakota Animal Disease Research and Diagnostic Laboratory
http://www.sdstate.edu/vs/adrdl
X
Texas A&M Veterinary Medical Diagnostic Laboratory
http://tvmdl.tamu.edu
X
Texas A&M Gastrointestinal Laboratory
http://vetmed.tamu.edu/gilab
X
Vita-Tech
www.vita-tech.com/dnabasedtests.cfm
X
Veterinary Molecular Diagnostics, Inc.
www.vmdlabs.com
X
Washington Animal Disease Diagnostic Laboratory
www.vetmed.wsu.edu
X
X
Wisconsin Vet Diagnostic Laboratory (emphasis in Avian)
www.wvdl.wisc.edu
X
Wyoming State Vet Lab
http://wyovet.uwyo.edu
X
Zoologix
www.zoologix.com/dogcat/Menu.htm
X
X
Statistics
Percentage of labs that use conventional and real-time PCR
31%
Percentage of labs that use conventional PCR only
64%
Percentage of labs that use real-time PCR only
5%
Simultaneous Testing of Multiple Pathogens
Indications for Use of Polymerase Chain Reaction Assays for Infectious Diseases
Molecular Biology Technologies
The Polymerase Chain Reaction in Veterinary Molecular Diagnostics
Comparing Real-Time Polymerase Chain Reaction with Traditional Polymerase Chain Reaction Testing for Diagnostic Applications
Preanalytic Variables
Nucleic Acid Extraction
Interpretation of Results
Reporting of Molecular Results
Regulatory Considerations of Molecular Laboratories
Guidelines for Clinicians to Select Molecular Diagnostic Laboratories
Summary
Molecular Testing for Infectious Diseases in Horses
Sample Submission
Pathogen
Tissue Submission
Anaplasma phagocytophilum
Whole blood
Babesia caballi
Whole blood
Clostridium difficile (antigen and toxin A and B)
Feces
Corynebacterium pseudotuberculosis
Aspirate from abscess, body fluid
Cryptosporidium spp.
Feces
Equine arteritis virus
NPS, uterine swab, placenta, aborted fetus
Equine coronavirus
Feces
Equine herpesvirus 1
NPS, whole blood, TW, BAL, CSF, CNS, uterine swab, placenta, aborted fetus
Equine herpesvirus 2
NPS, whole blood, TW, BAL
Equine herpesvirus 4
NPS, TW, BAL
Equine herpesvirus 5
NPS, whole blood, TW, BAL
Equine influenza virus
NPS, TW, BAL
Equine rhinitis A virus
NPS, urine
Equine rhinitis B virus
NPS
Equine rotavirus
Feces
Lawsonia intracellularis
Feces, intestinal biopsy
Leptospira spp.
Urine, blood, uterine swab, placenta, aborted fetus
Neorickettsia risticii
Whole blood, feces
Neospora hughesi
CSF, CNS
Rhodococcus equi
TW, BAL, feces
Sarcocystis neurona
CSF, CNS
Streptococcus equi subsp. equi
NPS, NPL, GPL, lymph node aspirate
Salmonella spp.
Feces
Theileria equi
Whole blood
West Nile virus
Whole blood, CSF, CNS
Clinical Applications
Respiratory Pathogens
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Molecular Diagnostics in Large Animals
Chapter 29
