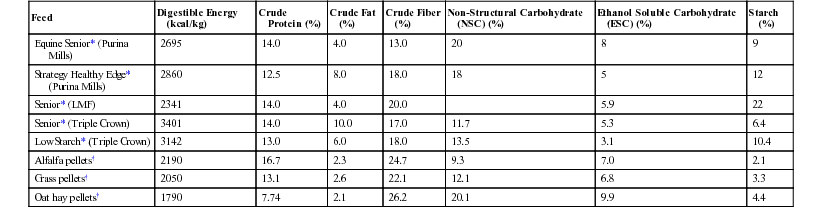
Meri Stratton-Phelps, Consulting Editor Raymond W. Sweeney The influence of nutrition on the recuperation of veterinary patients is often overlooked, although the effects of nutritional status on recuperative ability are well known. Many patients are in a state of protein calorie malnutrition when first presented to the clinician. Early intervention with supplemental calories, protein, and other essential nutrients provides the patient with the dietary resources needed to optimize immune function, promote wound healing, and improve the recovery of the animal. Multiple research studies have demonstrated that the immune response of an animal is directly related to the nutritional state of the animal.1–4 A deficiency of calories, protein, minerals, or vitamins alters the production of inflammatory cytokines, adversely affects leukocyte function, and decreases host resistance to bacterial infections.1,2 Clinical studies in hospitalized horses have demonstrated an improved recovery after gastrointestinal surgery in patients supplemented with intravenous nutrition.5 Different forms of diet therapy may be used, depending on the clinical condition of the animal and the ability of the animal to tolerate different types of supplemental nutrition. Enteral nutrition (EN) is the preferred method of nutrition support because it provides nutrients directly to enterocytes in a physiologically normal manner. EN can be provided to large animal patients that have a functional gastrointestinal tract and can tolerate placement of a nasogastric (NG) or esophageal feeding tube. Intravenous nutrition can be provided by adding dextrose to a patient’s intravenous fluids to provide energy, or supplemental nutrients can be administered in a parenteral nutrition (PN) formulation. PN may be the only suitable long-term (longer than 4 days) nutrition support choice for animals that cannot tolerate placement of an NG tube, that do not have a functional gastrointestinal tract, or that are recumbent. Before initiation of dietary therapy, the large animal patient must be examined to determine its nutritional needs. Animals may be anorectic owing to systemic disease, or they may be dysphagic owing to a mechanical (foreign body, abscess, poor dentition) or neurologic (botulism, tetanus, viral encephalitis) disease. Assessment of the nutritional status of the patient should include a measurement of the body weight (BW) and body condition score (BCS) of the animal. BW measurements should be taken at the time that the large animal patient is presented to the clinician and as frequently as possible during hospitalization. If a scale is not available, estimations of BW can be made with a weight tape or by using length and girth measurements (see Chapter 9). The BCS also helps in the evaluation of the nutritional status of the patient. The BCS system enables the clinician to subjectively assess the endogenous protein and lipid stores in a large animal patient. A list of BCS descriptions for different species is provided in Chapter 9. Changes in weight or BCS are often easily overlooked in day-to-day observations of the patient if a concerted effort is not made to detect them. Palpation of the animal (ribs, dorsal vertebral processes) is necessary in sheep with a heavy fleece, camelids with long fiber, and horses with a thick winter hair coat. Animals with a low BCS (1 to 3 of 9; 1 to 1.5 of 5) have minimal protein and lipid stores and are at greater risk for developing protein calorie malnutrition after a period of anorexia. Large animal patients with a high BCS (7 to 9 of 9; 3.5 to 5 of 5) that are anorectic may have an increased risk for developing complications (hyperlipemia, hepatic lipidosis) from abnormal lipid metabolism. A clinician should initiate dietary therapy in a large animal patient that loses 3% to 5% of its initial BW or whose BCS diminishes by 1 grade or more. Biochemical tests provide another method to evaluate the nutritional status of the large animal patient. Endogenous protein catabolism is a normal physiologic response to anorexia in animals. To date, few biochemical tests are available to assess protein malnutrition in large animals. Although anemia and hypoproteinemia (hypoalbuminemia) are occasionally seen in cases of malnutrition, they are not specific and frequently are associated with another primary disease process such as parasitism or protein-losing enteropathy. Severe protein malnutrition can result in abnormally low serum urea nitrogen (SUN) concentrations in horses and ruminants. Liver disease also decreases the formation of urea nitrogen and must be ruled out when evaluating the animal. Protein malnutrition can result in an increase in the urinary excretion of 3-methylhistidine, a myofibril amino acid that is not metabolized. Measurement of this metabolite in the future may be a useful tool to monitor protein catabolism in large animal patients.6 Underfed horses develop a mild hyperbilirubinemia (unconjugated) that, although not directly related to nutritional status, is readily reversed when food intake resumes.7,8 Animals that are in negative energy balance rely on endogenous stores of lipid as an energy source. Serum non-esterified fatty acids (NEFAs) and/or triglycerides are expected to rise in these animals. Once nutritional support or refeeding is initiated, these lipid metabolites usually decrease. Pathogenic elevations in serum triglyceride (greater than 500 mg/dL) develop in some equine patients that are anorectic and that have a high daily energy requirement (lactation, pregnancy), but hypertriglyceridemia can also occur in patients with insulin resistance, renal failure, obesity, and in predisposed breeds (miniature horses, ponies). Ketone bodies have a glucose-sparing effect in the body and are normally produced from fatty acids and amino acids in animals that are in negative energy balance. Ketonuria develops after the excess production of ketones and can be used to indirectly monitor the severity of caloric malnutrition in ruminants. Hypoglycemia is a common complication in large animal neonates that are malnourished but is an uncommon finding in adults. Both adult and neonatal large animals may develop glucose intolerance and hyperglycemia during periods of systemic illness and may require treatment with a diet that will not exacerbate the hyperglycemia. Electrolyte and mineral derangements including hypocalcemia, hypomagnesemia, hyponatremia, hypochloremia, hypophosphatemia, and hypokalemia may develop in inappetent, systemically ill large animals or during the refeeding process.9,10 Chronically malnourished animals must be closely monitored, and biochemical signs of the refeeding syndrome should be managed with appropriate electrolyte supplementation. Although it is generally accepted that sick animals have increased nutritional requirements, these requirements have not been quantified for specific disease conditions. Therapeutic nutrition is designed to provide adequate caloric intake during a period of hypophagia. Both overfeeding and undernutrition should be avoided when nutritional support is offered.11 Currently, the best estimation of energy and protein requirements for hypophagic horses and ruminants is calculated from the animal’s BW (see Chapter 9) or they can be determined from the National Research Council (NRC) tables.12 For adult horses, the resting digestible energy (DE) requirement is calculated as DErest (Mcal) = 0.975 + 0.021 BW (kg), and maintenance crude protein (CP) requirement is calculated as CP (g) = 1.26 BW (kg).12 For a 450-kg horse, these would equate to a 10.4-Mcal resting energy requirement and a 567-g crude protein requirement. The ideal BW should be used for calculating the energy requirement of an obese animal. Protein requirements can be calculated using an obese animal’s current BW as long as protein restriction is not required based on the patient’s clinical disease. Indirect calorimetry provides the best estimation of a patient’s resting energy requirements, but currently is only available for critically ill neonatal foals who have an estimated resting energy expenditure of 40 to 50 kcal/kg BW/day.13,14 Older foals can be fed between 70 and 100 kcal/kg BW/day. Dietary protein for foals ranges between 2 and 5 g protein/kg BW/day. For calves, the resting energy requirement is approximated as DE (Mcal) = 0.07 BW (kg) and digestible protein as DP (g) = 3.5 BW (kg), which would equate to 3.50 Mcal (3500 kcal) and 175 g of protein for a 50-kg calf. These calculated values represent starting points for formulating dietary therapy, and adjustment based on clinical response or specific medical conditions may be necessary. Vitamin and mineral requirements, also available from NRC tables, can usually be met if an enteral diet is formulated with commercial complete feed pellets or pelleted hay. Parenteral solutions can be supplemented with vitamins and minerals. Although B-vitamin deficiencies do not occur naturally in horses and cattle, supplementation may be beneficial in large animals with gastrointestinal diseases that result in the disruption of the normal tract flora that produce B vitamins. The animal’s feed intake should be monitored during treatment. If the animal is losing condition despite consuming all feed offered, more or higher-quality feed should be provided. If the animal’s appetite is poor, the patient should be offered a variety of highly palatable feeds, including fresh grass, dried forages, and complete commercial feed pellets. Although sweet feeds are palatable, they contain a high concentration of soluble carbohydrates (sugar, starch) and their use should be limited to a top dressing of other feeds. Ruminants in particular may consume small quantities of fresh feed if it is offered frequently, whereas if the same quantity is offered in one feeding, it may be ignored after a few bites. Many dairy cows can be coaxed into eating hay if it is placed in the back of the pharynx by the clinician, and oropharyngeal stimulation may result in increased voluntary feed consumption. Fresh silage and dried brewer’s grain frequently appeal to the hypophagic cow. Many sick horses and ruminants benefit from grazing if grass is available. A hospital feeding chart should be created to facilitate monitoring of the feed consumed by a hospitalized patient. Use of an inexpensive farm scale is the most accurate way to measure the amount of feed offered to a patient. Feed that is not consumed within a few hours should be weighed and discarded to prevent the accumulation of stale, and possibly fermented, malodorous feed. If the animal does not voluntarily consume enough feed to meet 75% of its resting energy requirements or 75% of its maintenance protein requirements, or if the animal loses weight or condition, dietary therapy with a liquid diet by intragastric administration should be considered. Dysphagic animals can also be managed with a tube-fed liquid diet as the sole source of nutrition. Enteral feeding (EF) with a liquid diet should be used in horses that require nutritional support to maintain their energy and protein intake during a short period (2 to 14 days) of partial or complete anorexia. Horses that are good candidates for EF do not have gastrointestinal ileus or gastric reflux and are able to tolerate an indwelling NG tube, repeated intubation, or an esophagostomy tube. Horses fed using EF should be standing. Diet choice will depend on product availability and nutrient requirements. Energy, protein, vitamin, and mineral requirements should be calculated for each equine patient to ensure that the appropriate concentration of nutrients is administered. Enteral diets may be administered to provide partial or complete nutrient supplementation to an adult horse. Liquid diets may be classified into four categories: (1) commercial equine enteral diets, (2) complete feed blender diets consisting of liquefied or finely ground whole food suspended in water, (3) composition diets containing highly digestible whole protein (usually casein or soy), fat, and carbohydrate, and (4) commercially available liquid enteral diets sold for human use. Because of the large variety of equine enteral diet options, human formulations are the least desirable choice for EF and should only be used when other diet options are not available. Commercial equine enteral diets offer a convenient option for managing a horse that requires supplemental EN. WellSolve Well-Gel (Purina Mills, LLC, St. Louis, Mo.) and Enteral Immunonutrition Formula (Platinum Performance, Inc., Buellton, Calif.) are two diets currently available for use. Both diets are mixed with water and can be administered through a small-diameter nasogastric tube. These diets can also be fed orally while a horse is being transitioned to a regular ration. Diets should be introduced gradually and fed in multiple small meals throughout the day. Blenderized equine commercial complete feeds or hay pellets are inexpensive ($5 to $10/day), and the ingredients are usually available at most veterinary hospitals or barns. Most products contain 14% to 25% crude fiber (dry matter [DM]) and vary in energy density from 2.6 to 3.1 Mcal/kg of diet. A list of commercial feeds that can be fed as a liquid enteral diet is provided in Table 50-1. Feeds with less than 18% non-structural carbohydrates (NSC) are preferred. Between 3.4 and 6.0 kg of diet per day is required to meet the resting energy requirements of a 450-kg horse. In some equine patients, protein supplementation may be required. If tolerated, a small volume of oil (113 to 227 mL) can also be added to increase the energy density of the diet. If a plain hay pellet is used, vitamin and mineral supplementation should be considered. This type of a diet may be prepared either by grinding one quarter to one third of the daily requirement of dry pellets in a blender and suspending the blended product in water or by soaking the pellets in water before blending to create a slurry. Each meal should be made fresh. The total amount of water that is added to the preparation should be recorded and added to the daily fluid administration log for the patient. The total daily volume of the diet should be divided into three to six meals and fed throughout the day. If only partial supplementation is required, or if a horse is being managed in a field setting, a smaller volume can be administered twice a day. A gradual introduction to feeding a blenderized diet is important. TABLE 50-1 Equine Feeds Suitable for Use in a Liquid Enteral Diet * As fed, manufacturer analysis. † As fed, average values, Equi-Analytical Laboratories, 2013. An ingredient-based composition diet can be designed by combining finely ground forage to provide structural carbohydrates with a concentrated source of protein. One such diet (Table 50-2) composed primarily of dehydrated cottage cheese, dextrose, and alfalfa meal requires considerable preparation by the clinician.15 Oil (113 to 227 mL) can be added as an energy source if the horse is not hyperlipemic. The omega-3 and omega-6 content of the oil should be considered when formulating a ration to provide for the nutritional management of inflammation. Any enteral diet that contains a high concentration of oil, dextrose, or NSC should be avoided. Structural carbohydrates should comprise 50% to 75% of the diet DM. The approximate cost for complete supplementation with the homemade equine diet is $40 to $50/day for a 450-kg horse. As indicated in Table 50-2, the homemade diet is introduced gradually over a period of 7 days. TABLE 50-2 Suggested Feeding Regimen for a Liquid Diet for a 450-kg Adult Horse15*
Nutrition of the Sick Animal

Assessment of Nutritional Status
Nutrient Requirements of Large Animals during Clinical Illness
Oral Supplementation
Liquid Diets for Horses
Adult Horses
Types of Enteral Diets.
Feed
Digestible Energy (kcal/kg)
Crude Protein (%)
Crude Fat (%)
Crude Fiber (%)
Non-Structural Carbohydrate (NSC) (%)
Ethanol Soluble Carbohydrate (ESC) (%)
Starch (%)
Equine Senior* (Purina Mills)
2695
14.0
4.0
13.0
20
8
9
Strategy Healthy Edge* (Purina Mills)
2860
12.5
8.0
18.0
18
5
12
Senior* (LMF)
2341
14.0
4.0
20.0
5.9
22
Senior* (Triple Crown)
3401
14.0
10.0
17.0
11.7
5.3
6.4
Low Starch* (Triple Crown)
3142
13.0
6.0
18.0
13.5
3.1
10.4
Alfalfa pellets†
2190
16.7
2.3
24.7
9.3
7.0
2.1
Grass pellets†
2050
13.1
2.6
22.1
12.1
6.8
3.3
Oat hay pellets†
1790
7.74
2.1
26.2
20.1
9.9
4.4
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


