Chapter 12 Mixed Acid-Base Disorders
A mixed acid-base disturbance is characterized by the presence of two or more separate primary acid-base abnormalities occurring in the same patient. An acid-base disturbance is said to be simple if it is limited to the primary disturbance and the expected compensatory response. Box 12-1 shows a classification of mixed acid-base disorders.
Box 12-1 Classification of Mixed Acid-Base Disorders
Patients with long-standing conditions may have a simple acid-base disorders. Those patients are at higher risk of developing a mixed acid-base disturbance when the disease progresses, when they develop complications, or when they are treated with drugs that interfere with acid-base status (e.g., furosemide). Box 12-2 shows examples of potential causes of such preexisting conditions.
Box 12-2 Examples of Potential Preexisting Disease Process Associated with Chronic Acid-Base Disorders*
Respiratory Acidosis
Compensation
The definition of a simple acid-base disturbance includes both the primary process causing changes in Pco2 or [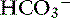 ] and the compensatory mechanisms affecting these measurements. A primary increase or decrease in one component (e.g., Pco2 or [
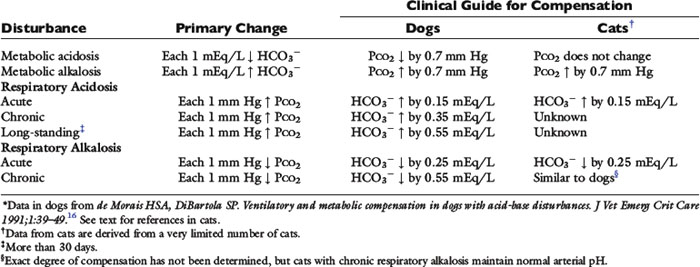
] and the compensatory mechanisms affecting these measurements. A primary increase or decrease in one component (e.g., Pco2 or [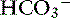 ]) is associated with a predictable compensatory change in the same direction in the other component (Table 12-1). Lack of appropriate compensation is evidence of a mixed acid-base disorder. Unfortunately, the magnitude of expected compensation in a given clinical situation is not known with certainty, and data in dogs have been derived mainly from experiments using normal dogs16 (Table 12-2). Compensatory rules for cats should be used with caution because values are derived from a limited number of normal cats with experimentally induced acid-base disorders. The reader is referred to Chapters 9, 10, and 11 for further discussion of compensation.
]) is associated with a predictable compensatory change in the same direction in the other component (Table 12-1). Lack of appropriate compensation is evidence of a mixed acid-base disorder. Unfortunately, the magnitude of expected compensation in a given clinical situation is not known with certainty, and data in dogs have been derived mainly from experiments using normal dogs16 (Table 12-2). Compensatory rules for cats should be used with caution because values are derived from a limited number of normal cats with experimentally induced acid-base disorders. The reader is referred to Chapters 9, 10, and 11 for further discussion of compensation.
Table 12-1 Primary and Secondary Changes in Simple Acid-Base Disorders
| Disorders | Primary Change | Compensatory Response |
|---|---|---|
| Metabolic acidosis | ↓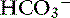 | ↓ Pco2 |
| Metabolic alkalosis | ↑ 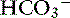 | ↑ Pco2 |
| Respiratory acidosis | ↑ Pco2 | ↑ 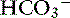 |
| Respiratory alkalosis | ↓ Pco2 | ↓ 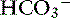 |
Respiratory compensation in metabolic processes
Metabolic acidosis is characterized by an increase in [H+], a decrease in serum [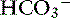 ] and blood pH, and a secondary decrease in Pco2 as a result of secondary hyperventilation. The expected decrease in Pco2 in dogs with metabolic acidosis may be estimated as 0.7 mm Hg for each 1-mEq/L decrease in [
] and blood pH, and a secondary decrease in Pco2 as a result of secondary hyperventilation. The expected decrease in Pco2 in dogs with metabolic acidosis may be estimated as 0.7 mm Hg for each 1-mEq/L decrease in [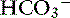 ].16 Cats with experimentally induced metabolic acidosis consistently show a lack of ventilatory compensation. In one study in which cats were chronically fed a diet containing NH4Cl, significant decreases in pH and [
].16 Cats with experimentally induced metabolic acidosis consistently show a lack of ventilatory compensation. In one study in which cats were chronically fed a diet containing NH4Cl, significant decreases in pH and [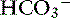 ] were observed, but there was no change in Pco2.9 Similar results were obtained in another study also adding NH4Cl to the diet31 and with dietary phosphoric acid supplementation.19 Contrary to what happens in dogs and humans, the feline kidney apparently is unable to adapt to metabolic acidosis and does not increase production of ammonia or glucose from glutamine during acidosis.31 Based on these studies, cats may not compensate for metabolic acidosis to the same extent (if at all) as do dogs and humans. Thus formulas for dogs or humans should not be extrapolated for use in cats. The clinical finding of metabolic acidosis and normal Pco2 in a cat should not be interpreted as evidence of a mixed process until more data are available about respiratory compensation in cats.
] were observed, but there was no change in Pco2.9 Similar results were obtained in another study also adding NH4Cl to the diet31 and with dietary phosphoric acid supplementation.19 Contrary to what happens in dogs and humans, the feline kidney apparently is unable to adapt to metabolic acidosis and does not increase production of ammonia or glucose from glutamine during acidosis.31 Based on these studies, cats may not compensate for metabolic acidosis to the same extent (if at all) as do dogs and humans. Thus formulas for dogs or humans should not be extrapolated for use in cats. The clinical finding of metabolic acidosis and normal Pco2 in a cat should not be interpreted as evidence of a mixed process until more data are available about respiratory compensation in cats.
Metabolic alkalosis is characterized by a decrease in [H+], an increase in serum [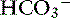 ] and blood pH, and a secondary increase in Pco2 as a result of compensatory hypoventilation. As a rule of thumb, a 1.0-mEq/L increase in plasma [
] and blood pH, and a secondary increase in Pco2 as a result of compensatory hypoventilation. As a rule of thumb, a 1.0-mEq/L increase in plasma [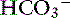 ] is expected to be associated with an adaptive 0.7-mm Hg increase in Pco2 in dogs with metabolic alkalosis.16 Little is known about respiratory compensation in cats with metabolic alkalosis. In one study with 12- to 14-week-old kittens made alkalotic by selective dietary chloride depletion, a 1.0-mEq/L increase in plasma [
] is expected to be associated with an adaptive 0.7-mm Hg increase in Pco2 in dogs with metabolic alkalosis.16 Little is known about respiratory compensation in cats with metabolic alkalosis. In one study with 12- to 14-week-old kittens made alkalotic by selective dietary chloride depletion, a 1.0-mEq/L increase in plasma [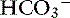 ] concentration was associated with a 0.7-mm Hg increase in Pco2.62 This value is remarkably similar to that observed in humans and dogs, but care should be exercised when extrapolating data from normal kittens to sick adult cats.
] concentration was associated with a 0.7-mm Hg increase in Pco2.62 This value is remarkably similar to that observed in humans and dogs, but care should be exercised when extrapolating data from normal kittens to sick adult cats.
Time is an important consideration when assessing compensation. Even in the experimental setting in which sudden changes in [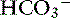 ] can be achieved, the respiratory response to acute metabolic acidosis in dogs occurs slowly, and it often takes 17 to 24 hours for maximal respiratory compensation to develop.16 Thus using the formulas within the first 24 hours of onset of metabolic acidosis may lead to an underestimation of the ventilatory response and the erroneous assumption that a mixed metabolic and respiratory disorder is present.
] can be achieved, the respiratory response to acute metabolic acidosis in dogs occurs slowly, and it often takes 17 to 24 hours for maximal respiratory compensation to develop.16 Thus using the formulas within the first 24 hours of onset of metabolic acidosis may lead to an underestimation of the ventilatory response and the erroneous assumption that a mixed metabolic and respiratory disorder is present.
Metabolic compensation in respiratory processes
Adaptive changes in plasma [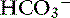 ] occur in two phases. In respiratory acidosis, the first phase represents titration of nonbicarbonate buffers, whereas in respiratory alkalosis, the first phase represents release of H+ from nonbicarbonate buffers within cells. This response is completed within 15 minutes (see Chapter 11). The second phase reflects renal adaptation and consists of increased net acid excretion and increased
] occur in two phases. In respiratory acidosis, the first phase represents titration of nonbicarbonate buffers, whereas in respiratory alkalosis, the first phase represents release of H+ from nonbicarbonate buffers within cells. This response is completed within 15 minutes (see Chapter 11). The second phase reflects renal adaptation and consists of increased net acid excretion and increased 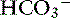 reabsorption (decreased Cl− reabsorption) in respiratory acidosis and a decrease in net acid excretion in respiratory alkalosis. Experimentally, renal adaptation requires 2 to 5 days for a chronic steady state to be established.21,46,51
reabsorption (decreased Cl− reabsorption) in respiratory acidosis and a decrease in net acid excretion in respiratory alkalosis. Experimentally, renal adaptation requires 2 to 5 days for a chronic steady state to be established.21,46,51
During acute respiratory acidosis, a compensatory increase of 0.15 mEq/L in [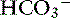 ] for each 1-mm Hg increase in Pco2 should be expected in dogs.16 There is a lack of data for compensation in cats with acute respiratory acid-base disorders, but values appear to be similar to those observed in dogs. In anesthetized, artificially ventilated cats made hypercapnic by exposure to increasing CO2 levels, the average compensatory increase in [
] for each 1-mm Hg increase in Pco2 should be expected in dogs.16 There is a lack of data for compensation in cats with acute respiratory acid-base disorders, but values appear to be similar to those observed in dogs. In anesthetized, artificially ventilated cats made hypercapnic by exposure to increasing CO2 levels, the average compensatory increase in [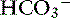 ] was 0.07 to 0.1 mEq/L for each 1-mm Hg increase in Pco2.24,54 In three awake cats exposed to an FIco2 of 4%,53 [
] was 0.07 to 0.1 mEq/L for each 1-mm Hg increase in Pco2.24,54 In three awake cats exposed to an FIco2 of 4%,53 [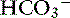 ] increased 0.16 mEq/L for each 1-mm Hg increase in Pco2, a value very similar to the one observed in dogs. During acute respiratory alkalosis, a compensatory decrease of 0.25 mEq/L in [
] increased 0.16 mEq/L for each 1-mm Hg increase in Pco2, a value very similar to the one observed in dogs. During acute respiratory alkalosis, a compensatory decrease of 0.25 mEq/L in [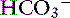 ] for each 1-mm Hg decrease in Pco2 should be expected in dogs.16 Compensation to hyperventilation has only been studied in anesthetized cats. The [
] for each 1-mm Hg decrease in Pco2 should be expected in dogs.16 Compensation to hyperventilation has only been studied in anesthetized cats. The [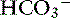 ] decreased an average 0.26 mEq/L for each 1-mm Hg decrease in Pco2, a value similar to that obtained in dogs.24
] decreased an average 0.26 mEq/L for each 1-mm Hg decrease in Pco2, a value similar to that obtained in dogs.24
In dogs with chronic respiratory alkalosis, a decrease of 0.55 mEq/L in [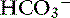 ] is expected for each 1-mm Hg decrease in Pco2.2,16 It is interesting to note that even in severe chronic respiratory alkalosis, the pH usually is normal. However, the normalization of pH in a clinical setting may take longer than 5 to 7 days. In humans with sustained respiratory alkalosis, the pH may not return to normal for 2 or more weeks.40 Cats chronically exposed to a hypoxic environment (FIo2 = 10%) for 28 days also were able to maintain a normal arterial pH.4 Expected compensation in cats cannot be inferred from this study, but based on the ability to maintain a normal pH, it may be reasonable to assume that cats can compensate for chronic respiratory alkalosis as well as dogs and humans. In dogs with chronic respiratory acidosis, serum [
] is expected for each 1-mm Hg decrease in Pco2.2,16 It is interesting to note that even in severe chronic respiratory alkalosis, the pH usually is normal. However, the normalization of pH in a clinical setting may take longer than 5 to 7 days. In humans with sustained respiratory alkalosis, the pH may not return to normal for 2 or more weeks.40 Cats chronically exposed to a hypoxic environment (FIo2 = 10%) for 28 days also were able to maintain a normal arterial pH.4 Expected compensation in cats cannot be inferred from this study, but based on the ability to maintain a normal pH, it may be reasonable to assume that cats can compensate for chronic respiratory alkalosis as well as dogs and humans. In dogs with chronic respiratory acidosis, serum [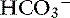 ] increases 0.35 mEq/L for each 1-mm Hg increase in Pco2.16 Similar rules have been used in humans with chronic respiratory acidosis, but these rules have been shown to work well in unstable, but not in stable, patients with long-standing respiratory acidosis.35 In this latter group of patients, a 0.51-mEq/L increase in [
] increases 0.35 mEq/L for each 1-mm Hg increase in Pco2.16 Similar rules have been used in humans with chronic respiratory acidosis, but these rules have been shown to work well in unstable, but not in stable, patients with long-standing respiratory acidosis.35 In this latter group of patients, a 0.51-mEq/L increase in [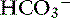 ] is expected for each 1-mm Hg increase in Pco2.35 Thus arterial pH appears to remain near reference ranges in human patients with long-standing respiratory acidosis.3 Similar results have been observed in dogs with chronic respiratory acidosis and no other identifiable reason for increased [
] is expected for each 1-mm Hg increase in Pco2.35 Thus arterial pH appears to remain near reference ranges in human patients with long-standing respiratory acidosis.3 Similar results have been observed in dogs with chronic respiratory acidosis and no other identifiable reason for increased [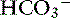 ] concentration other than renal compensation.22,25 Increases of 0.4525 to 0.57 mEq/L22 [
] concentration other than renal compensation.22,25 Increases of 0.4525 to 0.57 mEq/L22 [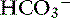 ] for each 1-mm Hg increase in Pco2 have been observed in dogs with chronic respiratory acidosis, suggesting that renal compensation in dogs with long-standing respiratory acidosis may return arterial pH to normal in stable patients.
] for each 1-mm Hg increase in Pco2 have been observed in dogs with chronic respiratory acidosis, suggesting that renal compensation in dogs with long-standing respiratory acidosis may return arterial pH to normal in stable patients.
Clinical approach
The first step is a careful history to search for clues that may lead the clinician to suspect the presence of acid-base disorders, followed by a complete physical examination. Urinalysis, routine serum chemistries, and electrolyte concentrations are useful, but confirmation of a mixed acid-base disorder requires blood gas analysis. After identifying the primary acid-base disorder (respiratory or metabolic), the expected compensation of the opposing parameter [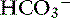 ] in a respiratory process; Pco2 in a metabolic process) should be calculated using the formulas in Table 12-2. A mixed acid-base disorder should be suspected when inappropriate compensation for the primary disorder is demonstrated. Compensation is said to be inappropriate if a patient’s Pco2 differs from expected Pco2 by more than 2 mm Hg in a primary metabolic process or if a patient’s [
] in a respiratory process; Pco2 in a metabolic process) should be calculated using the formulas in Table 12-2. A mixed acid-base disorder should be suspected when inappropriate compensation for the primary disorder is demonstrated. Compensation is said to be inappropriate if a patient’s Pco2 differs from expected Pco2 by more than 2 mm Hg in a primary metabolic process or if a patient’s [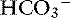 ] differs from the expected [
] differs from the expected [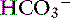 ] by more than 2 mEq/L in a respiratory acid-base disorder.2,16
] by more than 2 mEq/L in a respiratory acid-base disorder.2,16
Knowing that for each mEq/L decrease in [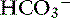 ] in a metabolic acidosis, Pco2 decreases 0.7 mm Hg (see Table 12-2), the expected compensatory change in Pco2 is estimated as:
] in a metabolic acidosis, Pco2 decreases 0.7 mm Hg (see Table 12-2), the expected compensatory change in Pco2 is estimated as:
where
Because the expected compensation has an error margin of ±2,
This patient has a Pco2 (24 mm Hg) that is more than 2 mm Hg lower than the minimal value for the expected Pco2 (28.4 mm Hg), indicating the presence of respiratory alkalosis in addition to metabolic acidosis. A similar line of thinking can be applied to calculate the expected compensation in other primary acid-base disorders. Some guidelines for adequate use of compensatory rules from Table 12-2 are expressed in Box 12-3. Some useful guidelines for quickly detecting mixed acid-base disorders in selected patients are shown in Box 12-4, whereas potential technical problems that may lead to misdiagnosing a mixed acid-base disorder are shown in Box 12-5.
Box 12-3 Guidelines for Adequate Use of Compensatory Rules from Table 12-2
Evaluation of the metabolic component of the acid-base disorder
Metabolic alkalosis can result from an increase in the strong ion difference (SID) caused by hypochloremia or by decrease in the concentration of total plasma weak acids [Atot] caused by hypoalbuminemia. Metabolic acidosis can be caused by a decrease in SID as a result of hyperchloremia or increased concentration of other strong anions (e.g., lactate, sulfate, β-hydroxybutyrate), or by an increase in [Atot] as a result of hyperphosphatemia. See Chapter 13 for further discussion of the role of albumin and phosphate in acid-base disorders.
Chloride Changes
Chloride is the most important extracellular strong anion. Increases in chloride lead to metabolic acidosis by decreasing SID, whereas decreases in chloride cause metabolic alkalosis by increasing SID. Therefore plasma [Cl−] and [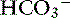 ] have a tendency to change in opposite directions in hypochloremic alkalosis and hyperchloremic acidosis. The contribution of [Cl−] to changes in base excess (BE) and [
] have a tendency to change in opposite directions in hypochloremic alkalosis and hyperchloremic acidosis. The contribution of [Cl−] to changes in base excess (BE) and [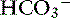 ] can be estimated by calculating the chloride gap, the chloride/sodium ratio, and the sodium-chloride difference (Table 12-3).
] can be estimated by calculating the chloride gap, the chloride/sodium ratio, and the sodium-chloride difference (Table 12-3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


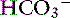 ] in blood. Respiratory alkalosis is that acid-base disorder resulting from a primary decrease in P
] in blood. Respiratory alkalosis is that acid-base disorder resulting from a primary decrease in P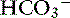 ] in blood.
] in blood.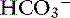 ] = 13 mEq/L, and P
] = 13 mEq/L, and P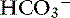 ] indicates that the primary process is a metabolic acidosis. The expected compensation is estimated assuming P
] indicates that the primary process is a metabolic acidosis. The expected compensation is estimated assuming P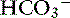 ] = 21 mEq/L as midpoint values. The change in [
] = 21 mEq/L as midpoint values. The change in [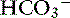 ] (
] (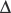 [
[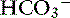 ]) is:
]) is: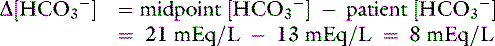
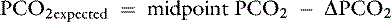
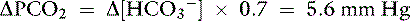
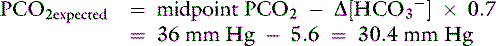
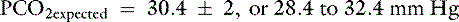
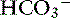 ] changing in opposite directions
] changing in opposite directions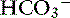 ])
])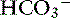 ] and P
] and P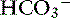 ]
]