Chapter 26 Enteral Nutrition
Nutritional assessment
Identification of patients needing nutritional support requires a thorough history, physical examination, and evaluation of laboratory data. A diet history is obtained to ascertain the quality, total daily intake, and appropriateness of the diet fed (Figure 26-1). Identify current drugs the animal may have been prescribed (e.g., corticosteroids, antibiotics, diuretics, cancer chemotherapeutic agents), as these drugs can affect nutritional homeostasis. To assess the need for nutritional support, the medical history includes inquiries about: involuntary weight loss, voluntary dietary intake, and presence of persistent gastrointestinal signs. Historical information suggestive of malnutrition includes rapid weight loss (greater than 10% of usual body weight); recent surgery or trauma; and increased nutrient losses from wounds, vomiting, regurgitation, diarrhea, or burns. Infection, trauma, burns, and surgery can increase nutrient needs, whereas prolonged use of antinutrient or catabolic drugs may result in nutrient depletion. The number of days an animal has not consumed adequate calories (hyporexia or complete anorexia) before hospitalization may be determined from the history.


Figure 26-1 Diet history form.
(From Buffington T, Holloway C, Abood S. Manual of veterinary dietetics. St Louis: Elsevier, 2004.)
Physical evaluation begins with the assignment of a body condition score,14 ranging from 1 (cachexic) to 5 (obese), with 3 being normal (Table 26-1). Other scoring systems have been developed for dogs and cats.59,60 Underweight and malnourished animals often have a body condition score less than 3 out of 5 because of loss of muscle mass and subcutaneous fat. Thin, dry skin, hair that is easily epilated, pressure sores, and poor wound healing may be seen, indicating the body has redirected its nutrient resources to support visceral protein synthesis at the expense of peripheral tissues.
Table 26-1 Body Condition Score
| Score | Classification | Description |
|---|---|---|
| 1 | Cachexic | Severely underweight, decreased muscle mass, no subcutaneous fat present, skeleton prominent. |
| 2 | Thin | Muscle mass adequate, little subcutaneous fat, skeleton apparent but not prominent. |
| 3 | Normal | Muscle mass adequate, ribs not seen but easily felt, obvious waist present when viewed from lateral or dorsoventral aspect. |
| 4 | Overweight | Individual ribs and spinous processes of vertebrae palpable only with moderate pressure, obvious fat pads present. |
| 5 | Obese | Palpation gives feeling of extensive fat cover over body. Large fat pads, respiratory and/or locomotor compromise. |
Many biochemical and hematologic abnormalities may occur during prolonged anorexia,62 including hypoalbuminemia, lymphopenia, and anemia, but they are not specific “markers of malnutrition.” Serum albumin concentration often is decreased in patients secondary to increased permeability of the vascular endothelium, as well as to decreased synthesis (or increased degradation) rates. Serum albumin concentration also is affected by hydration status and the presence of gastrointestinal, hepatic, or renal disease.51 Lymphopenia caused by malnutrition, stress, or immunosuppressive drugs. Starvation may interfere with immune competence even when the total lymphocyte count remains within the normal range.21
The main objective of nutritional assessment is to identify malnutrition as an independent problem. If not present initially, the animal should be periodically reevaluated during hospitalization to ensure that malnutrition does not develop secondary to an ongoing disease process, drug therapy, inability to eat, inappetence, or food deprivation.15 Nutritional support should be instituted in malnourished patients and in those for which voluntary food intake is impossible for prolonged periods.2
Evidence for early enteral nutrition
Numerous studies in human medicine have demonstrated multiple benefits to the early initiation of enteral nutrition (EEN), including improved gut barrier function, decreased bacterial translocation, and reduced septic complications and disease severity.* Early enteral nutrition in human medicine is defined as initiation of nutritional therapy within 48 hours of either hospital admission or surgery and is the standard of care.29 ASPEN guidelines recommend (Grade C) that enteral feedings should be initiated early within the first 24 to 48 hours following admission and advanced toward goal feeds over the next 48 to 72 hours in humans.70 In critically ill patients, nutritional support should commence as soon as the patient is hemodynamically stable, since many of these patients are already in a catabolic state. Although EEN remains controversial in dogs with acute pancreatitis, positive effects associated with EEN have been demonstrated in dogs diagnosed with parvoviral enteritis. Mohr, et al. randomized dogs with parvoviral enteritis into 2 groups: 15 dogs received no food until vomiting had stopped for 12 hours, and 15 dogs received early EN by nasoesophageal tube from 12 hours after admission.75 The EEN group demonstrated clinical improvements and had faster weight gains.75 Some veterinary nutritionists recommend nutrition support be instituted for any veterinary patient that is anorexic or anticipated to be hyporexic for longer than 3 days.
Nutrient needs
Dogs and cats that are eating require 50 to 100 mL of water per kilogram of body weight for daily maintenance, depending on environmental temperature, type of food, and amount of activity. Water requirements of normal fasting animals are only 5 to 10 mL/kg body weight per day (10% of the requirement of animals that are eating).83 This is because the solute load ingested with the diet and ultimately excreted by the kidneys is reduced.103
In sick animals, increased water losses via the urine may occur in some settings (e.g., diabetes mellitus, polyuric renal failure, hyperadrenocorticism, hyperthyroidism). Water also is lost in vomitus, diarrhea, burns, or hemorrhage. Insensible water losses (e.g., respiratory, cutaneous, fecal) may account for 20% to 40% of total water loss,5 which may be increased in the presence of fever, hyperventilation, hypermetabolism (e.g. sepsis), third-spacing of fluids and burn wounds.
Energy needs of anorexic patients are estimated by summing requirements for basal metabolic functions, activity, and the effects of disease. Basal metabolic rate (calories per day required for protein turnover and maintenance of ionic gradients across semipermeable membranes) is usually estimated by exponential method:3,46,54 97 x BWkgO.655 (Figure 26-2) or 70 x BWkgO.75. A linear method (30 x BWkg + 70) should only be used for animals weighing between 2 to 45 kilograms.46
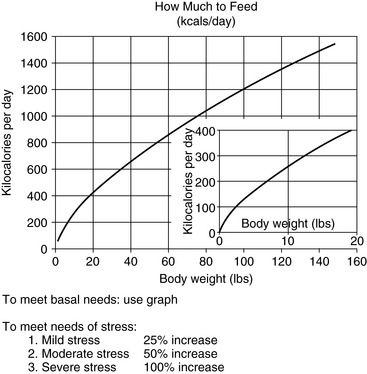
Figure 26-2 Approximate basal energy needs of dogs and cats.
(From Abood SK, Mauterer JV, McLoughlin MA, Buffington CA. Nutritional support of hospitalized patients. In: Slatter DH, editor. Textbook of small animal surgery, 2nd ed. Philadelphia: WB Saunders, 1993.)
We estimate the energy needs of our patients by assuming that those resting in a hospital cage have resting energy needs, determined from Figure 26-2. Although sepsis and burn injuries were found experimentally to increase energy expenditure 25% to 35% in dogs,101 more recent clinical studies found that the energy needs of resting critically ill, postoperative, and severely traumatized dogs were not higher than the basal needs of healthy animals.97 Based on these considerations and the complications associated with overfeeding, we determine initial estimates of energy needs on the basal requirement for the current body weight of the animal. Studies in humans suggest that metabolic rates greater than twice the basal requirement rarely occur, even in severely injured patients. In addition, overestimating nutrient needs increases the risk of the patient for problems associated with overfeeding.
The protein requirements of critically ill patients are not known. The protein requirements of young animals for growth are approximately 17% to 22% of total calories,87 and we use this guideline as an estimate for patients fed liquid-formula diets containing high-quality protein. Commercial pet foods may have lower protein quality and digestibility. If such diets are fed, higher protein concentrations (25% to 40% of calories) are required. We have used liquid diets containing 17% to 20% of kilocalories as protein in small animal patients with severe chronic renal failure successfully for short periods (<2 weeks), and have not found further restriction to be necessary. Patients with protein-losing diseases (e.g., protein-losing enteropathy or nephropathy) should have their estimated losses replaced. Protein losses by patients with protein-losing nephropathy are small relative to daily needs.19 In contrast, burned patients may lose significant amounts of protein, which may be replaced using diets containing higher percentages of kilocalories as protein.64
Hospitalized patients also require essential fatty acids, minerals, and vitamins. Specific vitamin and mineral needs depend on the type and severity of the underlying disease process. For short-term nutritional supplementation, at least sodium, chloride, potassium, phosphate, calcium, and magnesium should be provided. Provision of supplemental zinc should also be considered, especially in anorexic patients with gastrointestinal disease, where losses may be increased.102 Zinc also is important because of its role in protein synthesis, immune function, in vitro phagocytic activity, and taste and smell.82 Liquid enteral diets for nutritional support contain all the necessary minerals; so additional supplementation probably is not warranted. No studies have specifically evaluated the needs of veterinary patients for these nutrients. At present, provision of vitamins at or near the National Research Council requirements for growth seems reasonable in the absence of any specific contraindication77 (Table 26-2).
Appetite stimulation
Drugs used to stimulate appetite include the benzodiazepine derivatives, oxazepam (Serax, Wyeth, Madison, N.J.) and diazepam (Valium, Roche, Basel, Switzerland), the antiserotonergic agent, cyproheptadine (Periactin, Merck, Whitehouse Station, N.J.) and more recently the antidepressant agent, Mirtazapine (Remeron, Merck, Whitehouse Station, N.J.).66 However, it should be stressed that these drugs are only short-term options, and should not be used unless enteral nutrition support in the form of a temporary feeding tube is not an option. These medications should not be used for more than 24 to 48 hours in patients that are not consuming adequate nutrition.
Benzodiazepines are effective appetite stimulants in healthy dogs and cats.31 No controlled studies are available in veterinary patients for any of these compounds, which appear to be more effective for psychogenic than for pathologic anorexia. Psychogenic, or fear-induced, anorexia is common in hospitalized dogs and cats because of the strange surroundings, the stress of disease and trauma, and the unfamiliar human beings caring for them. Administration of 0.1 to 0.2 mg/kg body weight of diazepam intravenously, or 2.5 mg per cat per feeding of oxazepam orally, has been recommended to stimulate food intake in these settings.66 The recommended dosage of cyproheptadine for cats is 2 mg orally, two to three times daily. Although sometimes effective for psychogenic anorexia, these drugs do not appear to be effective for pathologic, disease-induced anorexia. Moreover, their sedative effects are undesirable in depressed animals, and they are contraindicated in patients with liver disease.95
Mirtazapine (Remeron) is a tricyclic antidepressant and an antiemetic in human medicine. It has been more recently used in canine oncology patients as an appetite stimulant at a dose of 15 to 30 mg by mouth once daily (cats can be dosed at 3.75 mg every 48 to 72 hours).58 In humans antidepressants are commonly tapered in consideration of antidepressant discontinuation syndrome, the significance of which has not been well characterized in pets, but its consideration may be advised.98
Other drugs, including prednisone (0.25 to 0.5 mg/kg body weight every other day) have been used to stimulate appetite.66 Although any of these drugs may be effective in isolated patients, none have been tested in controlled trials in veterinary medicine, and none have been demonstrated to be of consistent value. The greatest danger of pharmacologic appetite stimulation is that its use may give the false impression that adequate nutritional support is being provided. These drugs often stimulate animals to eat small meals immediately,66 and the clinician may conclude that food intake is adequate. Unless the total quantity of food eaten is measured, however, it cannot be determined if the animal continues to eat over the remainder of the 24-hour period. Use of these drugs should be restricted to animals in which food intake is being measured because of the inconsistent response to their use and the probability of delay of appropriate nutritional support.
Orogastric feeding tubes for neonatal nutrition
Neonates may be fed by passing a tube through the mouth or nose into the stomach for each feeding (Figure 26-3). To pass an orogastric feeding tube,24,35,94 first measure the distance from the mouth to the tenth rib and mark the tube with permanent marker. Lubricate the end of the tube with a water-soluble lubricant. Pass the tube through the mouth and into the pharynx. Hold the animal’s head at the normal angle of articulation to minimize the possibility of endotracheal intubation. When the animal swallows, advance the tube into the esophagus to the depth of the premeasured mark. Using limited restraint and opening the mouth just far enough to introduce the tube minimizes the animal’s objection to the procedure. Information on neonatal caloric requirements, commercial milk replacers, and instructions for feeding puppies30 and kittens39 from birth to weaning are available in written literature.13
Nasoenteric feeding tubes
Nasoenteric (nasoesophageal or nasogastric) feeding tubes should be placed for short-term (3 to 5 days) nutritional support in any patient that has not consumed or is not expected to consume adequate nutrition within 3 days of hospitalization. Techniques of nasoenteric tube intubation are available in written1,14,26,27 and video literature;14 the materials needed for this procedure are listed in Box 26-1. Nasoenteric feeding tubes are made in varying sizes and lengths, from a variety of materials by different manufacturers (Box 26-2). Polyvinyl chloride tubes are inexpensive and work well for intragastric feeding. They may harden if left in for prolonged periods, however, and should be changed approximately every 3 to 4 weeks. Polyurethane or silicone tubes are more expensive, but are resistant to gastric acid and may be used for prolonged periods. We choose the least expensive tube needed in the largest diameter and longest length that the patient can tolerate comfortably. Large-diameter tubes present less resistance to solution flow, whereas long tubes can be placed into the stomach, secured to the head, and still be attached behind an Elizabethan collar for easy access.
Box 26-1 List of Materials Needed for Nasoenteric Tube Placement
2. Stylet or guidewire (optional for tubes placed in large dogs, recommend the PTFE wire 0.35 × 180 cm or the hydrophilic 0.35 × 260 cm straight guidewires from Merit medical)
3. Topical anesthetic (Lidocaine) to desensitize nostril (divide dose by 3 and give each dose 5 minutes apart before tube placement)
4. Sterile, water-soluble lubricating jelly (Surgilube, Savage Laboratories, Melville, N.Y.) or lidocaine jelly 2% (Akorn, Inc., Buffalo Grove, Ill.) to lubricate the feeding tube and lubricate the guidewire.
5. Permanent marker to mark and adhesive tape to secure feeding tube
9. Suture material (2-0 or 3-0 Ethilon) to secure feeding tube
Box 26-2 Manufacturers of Products for Enteral Nutritional Support
A: feeding tubes (a: nasojejunal feeding tubes)
C: guide wires, placement devices, or placement kits
Abbott Laboratories—Animal Health [A, B, C]*
http://www.abbottanimalhealth.com/
http://abbottnutrition.com/ (Ross Products)
Bard Access Systems, Inc. [A, C; low profile devices]
Kendall Healthcare Products (Now part of Covidien) [A, a, C, D]*
http://www.kendallhealthcare.com/
MILA International, Inc. [A, a; low profile devices]*
Smiths Medical North America [A, B, C; gastrostomy tube introduction set]
Recommended tube sizes are listed in Box 26-1. A weasel-wire or guidewire/stylet (0.035 cm for 8 Fr or 10 Fr feeding tubes) can be used to assist passage of the tube (taking care that the wire/stylet does not stick out beyond the feeding tube). Sterile water soluble, lubricating jelly should be instilled into the feeding tube before introducing the stylet to facilitate easy removal of the stylet after the tube has been passed.
To pass a nasoenteric tube, a topical anesthetic is instilled into a nostril (4 to 5 drops of 0.5% proparacaine hydrochloride for cats or 2% lidocaine hydrochloride for dogs). The authors recommend repeating this step 2 to 3 times every 5 minutes before tube placement for adequate local analgesia. Most patients are depressed from their underlying disease and only require topical anesthetic. Based upon a recent prospective enteral nutrition study, 31 of 54 (57.5%) dogs required no sedation for feeding tube placement, 19 of 54 (35.1%) required mild sedation and 4 of 54 (7.4%) dogs had feeding tubes placed postoperatively while anesthetized.47 If sedation is required, a reversible opioid/benzodiazepine combination is recommended. Before passing the tube, measure the distance from the nostril to the stomach (approximately the caudal margin of the last rib at the level of the costochondral junction) and mark the tube with permanent marker. With the animal’s head held at the normal static angle of articulation to avoid tracheal intubation, the tube is passed through the nose of the patient by directing it caudomedially, then ventrally and caudally as the nasal planum is pressed upward (Figure 26-4). For doliocephalic dogs greater than 25 lb, pushing the tip of the nose upward in dogs, as the tube is passed, guides the tube into the ventral meatus.27 Slightly flexing the head downward may be helpful in directing the tube into the esophagus. For small dogs and cats, one should pass the tube directly through the ventral meatus without manipulating the nose. In other respects, the technique for cats is similar to that described for dogs.
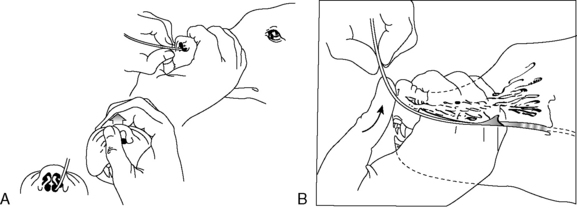
Figure 26-4 Position of head during nasogastric intubations.
(From Abood SK, Buffington CA. Improved nasogastric intubation technique for administration of nutritional support in dogs. J Am Vet Med Assoc 1991;199:577–579.)
Once the tube is installed, the stylet (if present) is removed and the position of the tube in the stomach or esophagus is checked for negative suction/gastric fluid. Placement should always be confirmed with a lateral/dorsal ventral cervico-thoracic radiograph. Many commercial feeding tubes have radiopaque markings to confirm the tube’s position. Once in place, the tube is sutured with a pursue string and Chinese finger trap suture pattern (Ethilon or silk suture) (Figure 26-5) or fixed with cyanoacrylate adhesive (Superglue) just lateral to the nostril in the nasal fold to avoid curling of the tube (Figure 26-6). Several knots should be placed at the first suture site and then a Chinese finger trap method applied for three to four throws to prevent movement of the tube. A second suture should be placed and secured to the lateral cheek (Figure 26-7). An Elizabethan collar is placed on all animals to prevent inadvertent tube removal (Figure 26-8). Topical lidocaine can be administered if the patient experiences retractable sneezing postplacement. Although uncommon, mild epistaxis may be seen if the nasal turbinates are irritated during placement.
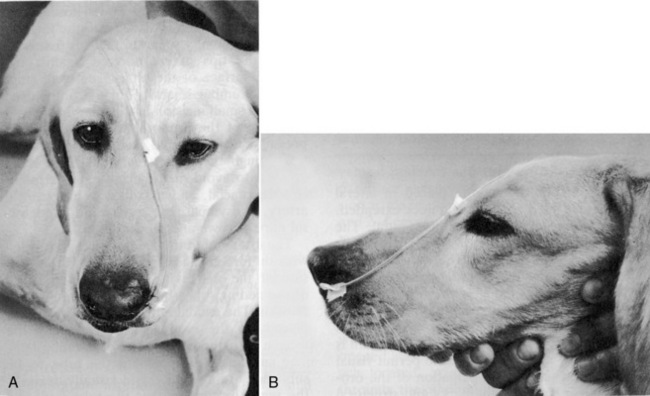
Figure 26-6 Technique for securing a nasoenteric tube. Tube should be secured as close to the nostril as possible.
(From Abood SK, Mauterer JV, McLoughlin MA, Buffington CA. Nutritional support of hospitalized patients. In: Slatter DH, editor. Textbook of small animal surgery, 2nd ed. Philadelphia: WB Saunders Co, 1993.)
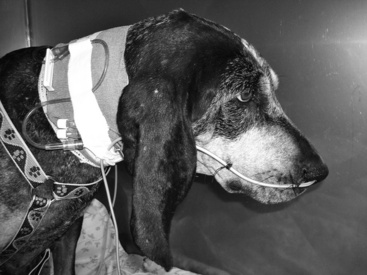
Figure 26-7 Canine patient with nasogastric enteral feeding tube in place sutured at both the nostril and cheek.
Nasogastric tubes also may be used for perioperative gastric reflux to remove gastric fluids and decrease the risk of regurgitation and aspiration pneumonia postoperatively. NE tubes may also be temporarily placed for administration of activated charcoal in acute toxicities.22 To prevent occlusion with food or mucus, NE feeding tubes should be flushed with water before and after each feeding, and kept securely capped so that water remains in the tube between feedings.
Esophagostomy feeding tubes
Esophagostomy tubes are specifically indicated for patients requiring prolonged feedings with at-home care or bypass of the oral cavity or oropharynx due to dysphagia, infection, inflammation, neoplasia, fracture, oronasal fistula, surgical procedures, or trauma.85,96 The diameter and length of the esophagostomy tube depends upon the size of the patient, type of diet, and personal preference. Soft red rubber urethral catheters or Silastic tubing are used commonly as esophagostomy feeding tubes; recommended feeding tube sizes are 12 to 16 Fr catheters for cats and dogs under 10 kg, and 12 to 20 Fr catheters for larger dogs (Stallion urinary catheters 6.6 mm × 137 cm (J-90s; Jorgensen Laboratories). The larger diameter feeding tubes (>14 Fr) permit feeding of pureed commercial pet foods. The distal end of esophagostomy tubes should not be placed through the lower esophageal high-pressure zone into the stomach. Studies have shown that such placement can cause gastroesophageal reflux by disrupting the integrity of this caudal esophageal high-pressure zone.25,63 Esophageal dysfunction, including abnormal clearing of acid within the distal esophagus, also may occur.61 In addition, chronic esophageal irritation by refluxed gastric acid may result in esophageal stricture formation. Placing the distal end of the esophagostomy feeding tube in the anterior or midthoracic region of the esophagus prevents mechanical disruption of the caudal esophageal high-pressure zone. Secondary peristaltic waves move the food bolus through the remainder of the esophagus into the stomach.
The necessary materials required for this technique are listed in Box 26-3. To place the esophagostomy tube, the patient is anesthetized and intubated (with either a short-acting injectable or inhalant anesthetic technique) and placed in right lateral recumbency with the head and neck extended. The hair is clipped from the lateral and ventral aspects of the neck using the vertical ramus of the mandible, the base of the vertical ear canal, and the caudal edge of the larynx as landmarks. The skin is aseptically prepared for surgery. The mouth is held open by an oral speculum to permit visual examination of the oral cavity and digital palpation of the oropharynx for surgical landmarks and structural abnormalities.
Box 26-3 List of Materials Needed for Esophagostomy Tube Placement
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree