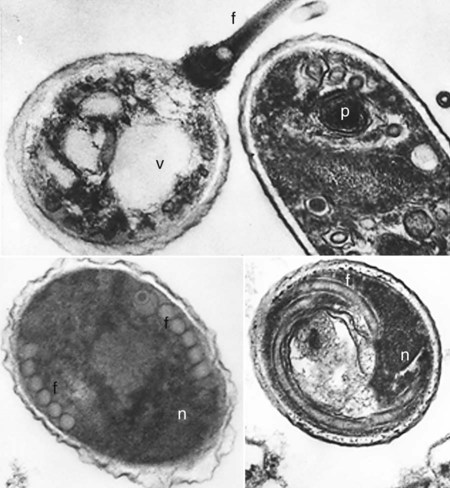
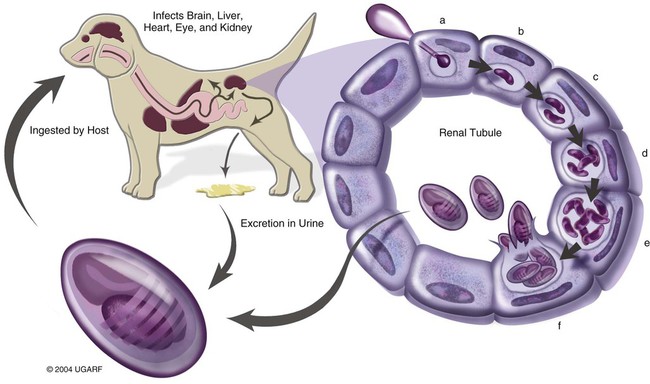
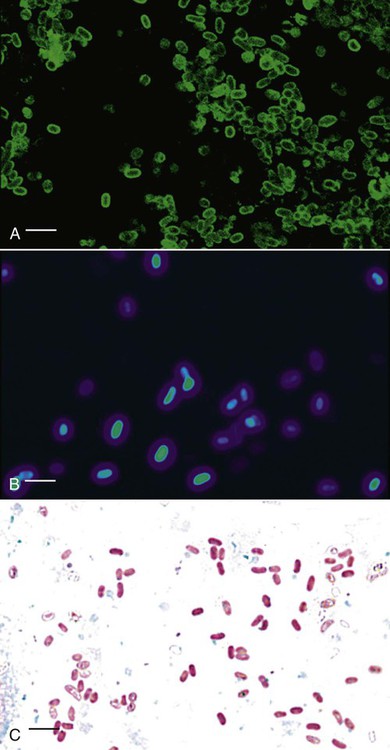
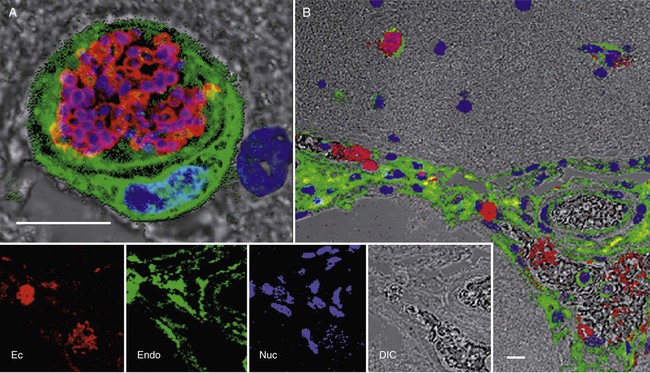
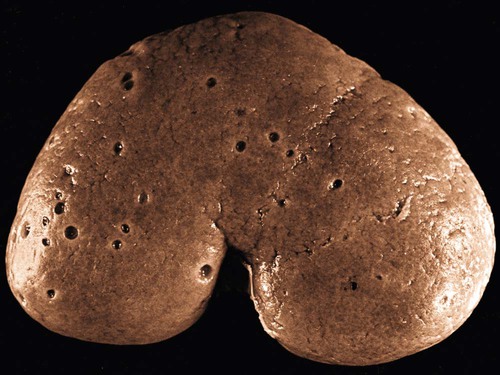
Peter J. Didier, Karen F. Snowden, Xavier Alvarez and Elizabeth S Didier* Microsporidiosis in dogs and cats is primarily caused by the obligate intracellular parasite Encephalitozoon cuniculi, which is a member of the phylum Microsporidia. Once considered to belong to the protozoa, molecular analysis has reclassified Microsporidia with the fungi.42,66 More than 1000 species of microsporidia-classified into approximately 100 genera-infect insects and members of all classes of vertebrates.16,32 Medically important species of the genus Encephalitozoon (Encephalitozoon hellem, Encephalitozoon intestinalis [previously named Septata intestinalis], and three strains of E. cuniculi)11,14,35,50,61 that have been described may infect dogs, cats, and birds as well as a wide range of wild, farm, and domestic animals.8,36,36 Another microsporidian species, Enterocytozoon bieneusi, a common intestinal opportunistic parasite in patients with the acquired immunodeficiency syndrome (AIDS), has been identified in stool samples from cats, cattle, dogs, nonhuman primates, and pigs* and in milk from dairy cattle.66,70 Although no disease from Enterocytozoon bieneusi or E. intestinalis has been described in dogs and cats, the zoonotic potential of these subclinical infections needs additional investigation.127 E. cuniculi is used as the representative microsporidial species for descriptive purposes in this chapter. Mature spores of E. cuniculi are small and oval, measuring approximately 1.5 µm wide and 2.5 µm long. They contain the distinctive coiled polar tubule or filament-and-extrusion apparatus that distinguish microsporidia from all other organisms (Fig. 69-1). The polar tubule is used to propel the sporoplasm (containing the microsporidian nucleus) into the host cell. Spores also contain a posterior vacuole, ribosomes, endoplasmic reticulum, Golgi-like membranes, and remnant mitochondria or mitosomes. The spore coat contains an outer glycoprotein coat, a middle layer containing chitin, and an internal plasma membrane.32 Infection of most mammalian hosts with E. cuniculi occurs by ingestion or inhalation of spores from contaminated urine or feces that are shed by infected hosts.16,33 Infection by transplacental transmission and traumatic inoculation has been reported as well.74,97 Once internalized, infectious spores invade host cells by propelling the sporoplasm through the everted polar tubule by a process called germination. The sporoplasm of E. cuniculi develops within a host-cell-derived membrane-bound parasitophorous vacuole (Fig. 69-2).89 The organisms undergo schizogony (also called merogony), which is an asexual process of cell division or binary fission. During sporogony and maturation, organisms develop the spore coat and organelles (the polar tubule, endoplasmic reticulum, and polar cap). The host cells eventually rupture and release organisms that infect new cells or environmentally resistant spore forms, which are shed in the urine or feces.16 Typical organs of localized infection in dogs and cats include the kidney, liver, and brain.108 Natural infections with E. cuniculi have been described in a wide range of hosts, including rabbits, mice, cats, dogs, foxes, and humans.16,17,74,110,123 Molecular genetic methods have been used to characterized the isolates into three genotypes. Of the three strains (genotypes) of E. cuniculi, strain I has been identified in rabbits and humans. Strain II has been identified in mice, rats, and blue foxes, and strain III has been found in dogs, swine, and humans.* Strain III has also caused fatal infections in nonhuman primates.6,47,60,88 Differences in these isolates have been confirmed by protein electrophoresis, Western blot immunodetection, and small-subunit ribosomal RNA (SSU rRNA) gene sequence analysis. Although epidemiologic information is sparse, one report suggests that E. cuniculi was transmitted to a dog groomer with AIDS.127 However, strain I (primarily from rabbits) can infect mice, cats, and sheep in experimental situations.96 A comparison of isolates from humans and rabbits suggests that the infecting strain is zoonotic.23,24 A strain of E. cuniculi from a dog produced a subclinical infection in immunocompetent monkeys.113 Relatively few isolates of E. cuniculi are available for comparison, so the host specificity of the E. cuniculi strains is still unclear. A few studies have been published on the prevalence rates of naturally acquired E. cuniculi infections.3,48 In a group of stray dogs that were housed three per cage in a London kennel, 13.3% of the dogs were found to express specific antibodies to E. cuniculi.52 In South Africa, a serologic study of 220 serum samples submitted for clinical evaluation suggested a prevalence of 18% in domestic dogs.105 Among 52 dogs with renal failure, 12 (23%) expressed specific antibodies for E. cuniculi, compared with 2 of 42 (5%) control dogs.104 Based on the presence of specific antibodies, a prevalence of 70% was also reported in 50 dogs housed in kennels. This high prevalence may have been a result of confinement and closer contact to contaminated urine or feces.105 In an urban animal shelter, 6 of 20 dogs were found to be excreting microsporidial spores in the stools.55 In additional serologic studies, the prevalence of reactive antibody titers to E. cuniculi in canine sera has ranged from zero (in Norway)3 to 14% (in Brazil),69 to 35% (in Colombia),69 to 37% (in Slovakia).48 Natural E. cuniculi or Enterocytozoon bieneusi infections of cats are rare and documented in a few reports.13,73,73 Subclinical infections are common in rabbits and rodents; however, occasional central nervous system or renal disease develops in rabbits.65 Overt illness has been most commonly reported in wild foxes and domestic dogs, with sporadic reports in other species.101 Clinically significant infections of E. cuniculi generally develop in neonatal and young puppies and are acquired by transplacental transmission and ingestion or inhalation of spores shed from the mother.52,76 Older dogs may become infected with microsporidia by inhalation or ingestion of spores from contaminated urine or feces or by ingestion of tissues from rabbits or mice infected with E. cuniculi.76 Experimental canine infections also may be transmitted by intraperitoneal inoculation, whereas kittens may be infected experimentally by intracerebral, intraperitoneal, or oral inoculation. Cats and older dogs generally show few or no clinical signs of disease but do sporadically shed organisms in the urine. Younger dogs infected with microsporidia have clinical signs associated with renal disease.104 In experimental studies of other mammals, microsporidiosis has been chronic and asymptomatic in healthy immunocompetent animals but severe and lethal in T-cell-depleted animals. Clinical signs usually develop in neonatal dogs, within a few weeks postpartum up to the weaning period of 6 weeks, or shortly thereafter. Several pups in a litter may have inappetence, stunted growth, and general unthriftiness.52,76,76 As infection progresses, animals show signs of renal failure and neurologic abnormalities such as mental depression, ataxia, convulsions, and blindness.18,75,75 Animals may develop aggressive behavior consisting of viciousness, biting, and abnormal vocalizations. The high frequency of anti–E. cuniculi antibodies in dogs with azotemia suggests that E. cuniculi may contribute to chronic renal disease in dogs.104 Clinical laboratory findings are available only for experimentally infected dogs.12,103,108,109 A normochromic, normocytic anemia is a consistent finding and may result from severe renal lesions with depression of erythropoietin production. In contrast, leukocyte numbers, especially those of lymphocytes and monocytes, are higher. Bone marrow is hypercellular, with a preponderance of large mononuclear cells. Serum biochemical findings include increased alanine aminotransferase and alkaline phosphatase activities (in the high normal to slightly elevated range), variable serum urea nitrogen and creatinine levels, and increased total serum protein levels. Cerebrospinal fluid may have more protein and cells in animals with behavioral and neurologic signs and higher anti–E. cuniculi IgG levels in the cerebrospinal fluid than in serum. Urinalysis may demonstrate hematuria and pyuria. Clinical signs in feline encephalitozoonosis vary. Severe muscle spasms, superficial corneal infection with blepharospasm, depression, paralysis, and death have occurred in experimental infections using E. cuniculi with terminal findings of encephalitis and nephritis.85 Prevalence of IgG antibodies to E. cuniculi was not significantly different between cats in Virginia, with or without chronic kidney disease.52a Serologic prevalence in cats with E. cuniculi is reported at 23.6%48 and 17% for Enterocytozoon. bieneusi.94 Scattered reports document Enterocytozoon. bieneusi in feline stool samples, but no clinical disease has been reported.1,12,73,94 Immunologically competent hosts produce specific antibodies to E. cuniculi, which can be detected by methods such as indirect fluorescent antibody staining and an enzyme-linked immunosorbent assay(ELISA).36 An indirect fluorescent antibody titer greater than 100 or an ELISA titer of 800 or greater is considered to be positive for the presence of antibodies to E. cuniculi.52,105 However, serologic tests are not commercially available, and some concerns exist about the diagnostic reliability of serologic tests in immunologically immature puppies or casually exposed hosts. ELISA kits for measuring antibodies in various species are available (see Web Appendix 6). The utility of serum agglutination tests to screen for anti-microsporidia antibodies using microsporidian spores has been demonstrated and could prove useful in a clinical setting when reagents become available.2,59 Therefore, diagnostic methods have focused on detecting the microsporidian spores in urine, stool, and tissue specimens. Transmission electron microscopy (TEM) has been considered the standard for the specific diagnosis of microsporidiosis.112 Presence of the polar tubule distinguishes microsporidia from other organisms (see Fig. 69-1).16 However, TEM is relatively insensitive, costly, and time consuming and requires technical expertise.30 Cytologic examination of body fluids is extremely important when making a clinical diagnosis in animals with disseminated infections. Spores shed into the urine from parasitized renal tubular epithelial cells are readily identifiable in the sediment with Gram or Ziehl-Neelsen staining.44,46 Stained spores are gram positive, whereas proliferative stages are gram negative. Microsporidia in stool specimens are also difficult to distinguish from other gram-positive bacteria; however, indirect fluorescence methods using monoclonal antibodies or hyperimmune polyclonal antisera can specifically identify microsporidia (Fig. 69-3, A).5,19,115,119 When viewed with cross-polarizing filters, Encephalitozoon species and other microsporidia have a birefringent appearance, unlike the coccidians Toxoplasma gondii and Isospora species. Chitin-staining fluorochromes (e.g., Calcofluor White 2MR, American Cyanamid, Princeton, NJ) are useful for detecting the microsporidia, which stain as white to turquoise oval halos when viewed with ultraviolet microscopy (see Fig. 69-3, B).* Microsporidia are easily stained with the modified trichrome stains (using 10-fold higher concentrations of chromotrope 2R) and appear bright pink with a diagonal pink band and a clear posterior vacuole. Bacteria stain with the counterstain (see Fig. 69-3, C).* Yeasts also stain bright pink but lack a posterior vacuole, and they are usually larger and more round than the oval microsporidia. Commercial testing using these methods is available through human hospital services currently (UTMB, Cleveland Clinic, Mayo Clinic, and many others). However, these staining methods typically cannot be used to discriminate between species of microsporidia. Molecular genetic identification by the polymerase chain reaction may soon replace TEM as the method of choice for confirmation of microsporidiosis because experimental studies suggest that PCR is significantly more sensitive and specific.† Based on experimental infections, gross lesions of canine encephalitozoonosis include hepatomegaly, petechiae on multiple organs, patchy consolidation and edema of the lungs, fibrinous pericarditis, regional enteritis, focal myocardial degeneration, swollen kidneys, hemorrhagic cystitis, and splenomegaly.108 The kidney may contain mild petechiae or severe cortical cysts and infarcts (Fig. 69-4). The brain may contain thrombosed meningeal blood vessels, focal encephalomalacia, and cystic spaces within the parenchyma. In naturally infected blue foxes, nodular thickening of the extramural coronary arteries and lymphadenomegaly have been seen.82 Histologically, dogs and blue foxes with encephalitozoonosis consistently have nonsuppurative meningoencephalitis (Fig. 69-5).4,75,82,101,112 Fibrinoid necrosis of small and medium-size arteries of the brain can result in vasculitis, thrombosis, encephalomalacia, and infarction. Parasitophorous vacuoles develop in endothelial and glial cells. Kidneys of dogs, cats, and foxes have multifocal nonsuppurative interstitial nephritis, sometimes with necrotizing inflammation in the pelvis.4 Parasitophorous vacuoles with organisms are present in the renal tubular epithelia. In the heart, focal myocardial necrosis, vasculitis, and fibrinoid necrosis of small and medium-size arteries are associated with E. cuniculi in endothelial cells and smooth muscle cells. In experimental infections, livers of dogs and cats developed vascular fibrinoid necrosis, focal hepatic necrosis, and lymphoplasmacytic infiltration associated with E. cuniculi in hepatocytes, Kupffer’s cells, and endothelial cells. In blue foxes, ocular lesions are attributable to arterial lesions of the short and long ciliary arteries and retinal vessels.82 Other nonspecific lesions include pulmonary edema, nonsuppurative interstitial pneumonia and enteritis, lymphadenomegaly, reticuloendothelial hyperplasia of the spleen, and hyperplasia of the bone marrow.121
Microsporidiosis
Etiology
Epidemiology
Clinical Findings
Dogs
Cats
Diagnosis
Pathologic Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine