CHAPTER 11 Microscopic Examination of the Urinary Sediment
The urine specimen has been referred to as a liquid tissue biopsy of the urinary tract, painlessly obtained (Haber, 1988). The routine urinalysis is composed of two major components—the macroscopic and the microscopic evaluations. The chapter will focus on findings that can be microscopically observed in the urinary sediment. The evaluation of the physical characteristics and interpretation of alterations detected by reagent test-strip methodologies can be found elsewhere (Meyer and Harvey, 2004). Microscopic examination of urine sediment should be conducted on every urinalysis even if no abnormalities are detected by the reagent test-strip. Studies indicate that up to 16% of urine samples with unremarkable reagent test-strip findings can have positive microscopic findings, notably pyuria and bacteriuria (Barlough et al., 1981; Fettman, 1987). The findings of another study support the recommendation to routinely examine microscopically and culture the urine of dogs with hyperadrenocorticism and diabetes mellitus since clinical signs of bacterial cystitis often are not present (approximately 95% of the cases) and bacteriuria or pyuria may not be observed (approximately 19% of the cases) (Forrester et al., 1999). Bacteria are never considered a normal finding in urine collected by percutaneous cystocentesis even when clinical signs of cystitis are absent (Forrester et al., 1999).
Free catch, catheterization, and percutaneous cystocentesis are the techniques used to obtain urine. The latter method is the surest way to avoid contamination. The specimen should be processed and examined within minutes of collection for the most accurate semiquantitative assessment of the findings. Casts are the most labile constituent and begin to lyse within 2 hours. Cells loose their integrity within 2 to 4 hours depending on the osmolality of the urine. Refrigeration of the urine specimen (for up to 6 hours) is a good way to preserve the physiochemical properties and crystals and delay cellular degeneration. Urine pH will be minimally affected if preserved by refrigeration for up to 24 hours (Raskin et al., 2002). Lowering the temperature of urine enhances crystal formation, resulting in an inaccurate semiquantitative assessment of their true numbers physiologically. A consistent volume of urine, generally 5 ml, should be routinely assessed so that the findings can be semiquantitated and compared to reference values as well as followed in a patient under treatment (Osborne and Stevens, 1999).
Following centrifugation of the urine specimen in a conical-tipped tube, most of the supernatant is decanted to leave an equal amount of sediment and urine. The sediment is resuspended by flicking the tube several times with the finger. One unstained drop is placed on a clean glass slide with a pipette, a coverslip is applied, and the specimen is examined. Subdued microscopic lighting is required to accentuate the elements in the sediment. The microscope’s condenser must be lowered and the iris diaphragm partially closed in order for the constituents to be most conspicuous (Fig. 11-1A). Phase-contrast microscopy accentuates the outline of even the most translucent constituents, simplifying the detection of casts and bacteria. Polarized microscopy is used to enhance the identification of crystals.
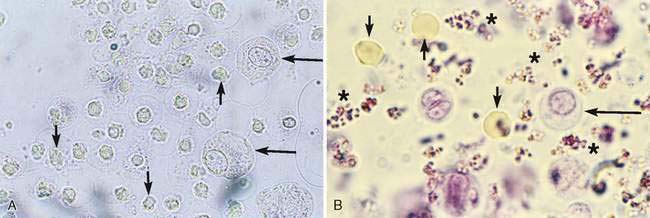
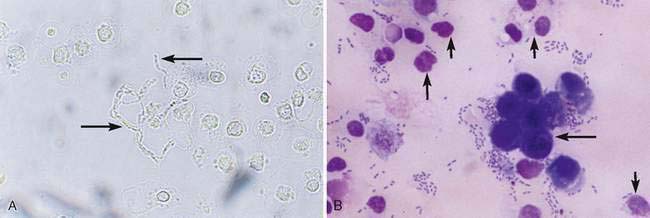
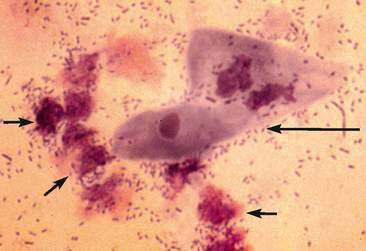
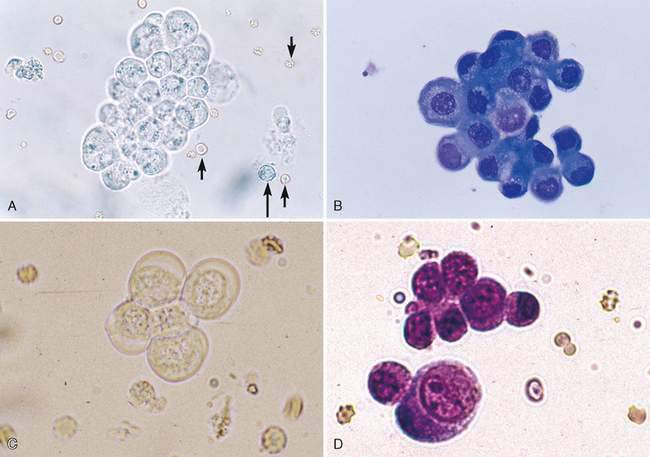
A water-based stain (Sedi-Stain, BD Clay Adams, Sparks, MD, or 0.5% new methylene blue) can be used to accentuate cellular detail. One drop of stain is added to the resuspended sediment, mixed by flicking the tube with the finger, allowed to incubate for 2 to 3 minutes, and one drop of the stained sediment placed on a clean glass slide with a pipette. Stain precipitate will develop over time in the bottle and microscopically appear similar to bacteria (Fig. 11-1B). When precipitate is observed, new stain must be employed or the current stain passed through a filter. It is good practice to initially examine an unstained specimen followed by the examination of a stained preparation when there are findings that require additional definition or as a learning tool. A cytology preparation of the sediment is another approach to characterize organisms (Figs 11-2 to 11-3), cells (Fig. 11-4A-D), or the composition of casts that are not readily recognized in an unstained preparation. One drop of unstained, resuspended sediment is placed near the frosted end of the slide and spread with another slide or a compression (squash) preparation is made, air-dried, and stained (refer to Chapter 1). Often the constituents will wash off during the staining process because of the low-protein nature of the specimen. Serum-coated slides should be used to “glue” the constituents in the sediment to the surface of the slide (refer to Chapter 1, Key Point).
Lipid droplets (Fig. 11-5A-C) will resemble cells but are generally more variable in size. Erythrocytes, leukocytes (see Fig. 11-2A), and casts (Figs. 11-6 to 11-10) are expressed as the number observed per high-power field (HPF, 40× objective) (Box 11-1). The matrix of all casts is formed by Tamm-Horsfall protein, a glycoprotein secreted by the ascending loop of Henle and possibly the distal tubule. See Meyer and Harvey (2004) for a more detailed discussion of Tamm-Horsfall protein. Crystals are expressed as few (occasional), moderate, or many per low-power field (LPF, 10× objective). The urine pH, temperature, and specific gravity affect the solubility of crystals (Graff, 1983). Struvite (Fig. 11-11) (magnesium ammonium phosphate; triple phosphates is a misnomer), amorphous phosphate (Fig. 11-12A), calcium phosphate (Fig. 11-12B), ammonium urate (Fig. 11-13A-C) (also referred to as ammonium biurate), and calcium carbonate (Fig. 11-14) have propensity to form in neutral pH or alkaline urine. Ammonium urate crystals are uncommon in healthy dogs and cats. An exception is ammonium urate crystalluria in apparently healthy Dalmatians and Bulldogs. Dalmatians have an in-born error of uric acid metabolism in which uric acid is not changed to allantoin and results in hyperuricosuria and the predisposition to ammonium urate and uric acid crystalluria. In other breeds, the water-soluble allantoin that is formed is excreted in the urine. Ammonium urate crystalluria is associated with congenital portosystemic vascular anomalies (shunts) and with liver insufficiency secondary to reduced hepatocellular mass (e.g., cirrhosis). Urine with a neutral or acidic pH tends to favor the formation amorphous urates (Fig. 11-15A), sodium urate (Fig. 11-15B-D), uric acid (Fig. 11-15E), calcium oxalate (Fig. 11-16A-E), bilirubin (Fig. 11-17A&B), cystine (Fig. 11-18), calcium hydrogen phosphate dehydrate (brushite) (Fig. 11-19), sulfa (Fig. 11-20A&B), and tyrosine (Fig. 11-21). A lignin test is used as a screening test for sulfonamides. To perform the test, place a few urine drops on a blank strip of newspaper followed by a drop of 25% hydrochloric acid in the center of the moist area. Yellow to orange reaction within 15 minutes indicates a positive reaction (Graff, 1983).
< div class='tao-gold-member'>