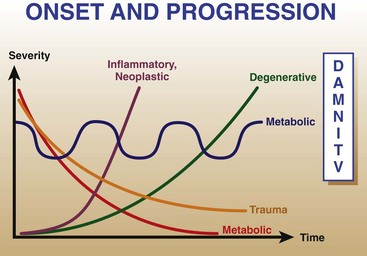
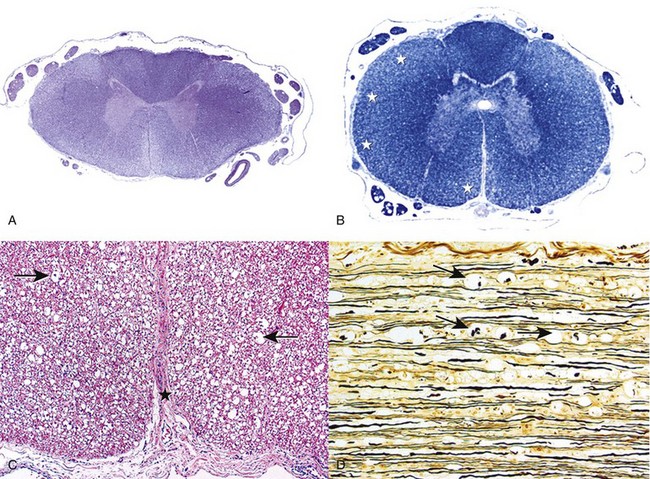
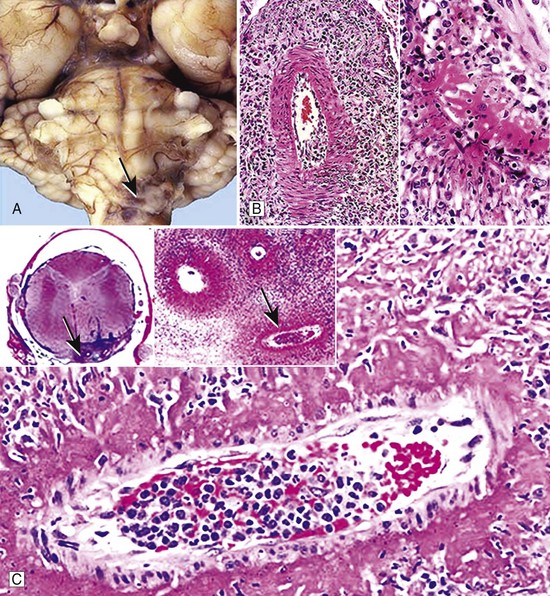
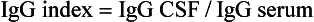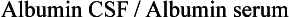Chapter 30 The clinician’s objective upon performing the neurologic evaluation in spinal cord disease is to determine the neuroanatomic localization (C1-C5, C6-T2, T3-L3, L4-S3) and lesion distribution (focal, multifocal, diffuse) of the spinal cord disorder. A complete description of the neurologic examination is presented in Chapter 26 and is beyond the scope of this chapter. Similar to other neurologic disorders, myelopathies may be classified according to the “DAMNIT V” (degenerative–anomalous–metabolic–neoplastic–nutritional–inflammatory/infectious–traumatic–toxic–vascular) scheme. Although overlap can be seen among the various categories of spinal cord disease, each is associated with a typical signalment, disease onset and progression, and lesion distribution within the nervous system (Figure 30-1, Box 30-1). The most common categories of spinal cord disease include degenerative, neoplastic, infectious and inflammatory, traumatic, and vascular disorders (see Box 30-1). Anomalous, metabolic, and nutrition-related conditions also are considered here but are less common causes of spinal cord dysfunction in dogs and cats. Neurologic signs can result from congenital malformations that involve the vertebral column, the spinal cord, or both.6 In patients with spinal cord malformations such as myelodysplasia (anomalies of the spinal cord resulting from incomplete closure or development of the neural tube), clinical signs typically are nonprogressive or slowly progressive from early in life. Vertebral malformations do not always affect the spinal cord and may be identified incidentally on radiographs or cross-sectional imaging. If present, neurologic signs associated with vertebral column malformations typically are recognized early in life and also are nonprogressive or slowly progressive. Occasionally, vertebral column malformations do not cause neurologic signs until later in life because of stenosis of the vertebral canal, progressive deformity, or instability. Metabolic disorders may affect animals of any age. The rate of onset of clinical signs may be variable but most commonly is subacute. An acute onset of signs is rare but may occur with storage disorders. Most metabolic conditions wax and wane over time. Diffuse, nonspecific signs or bilaterally symmetric deficits referable to the cerebrum or thalamus are the most common localizations. However, spinal cord signs may predominate early in the disease course as in canine polioencephalomyelopathies19,73 and some lysosomal storage disorders such as globoid cell leukodystrophy.46 Nutritional diseases affecting the nervous tissue are uncommon in small animals and rarely affect predominantly the spinal cord. Neurologic signs are typically bilaterally symmetric. Their onset is variable (subacute or insidious), and they typically are slowly progressive. Distribution can be diffuse or multifocal, as some nutritional diseases may affect selective areas of the central nervous system. Examples of nutritional disorders that may result in signs of spinal cord dysfunction include thiamine deficiency,104 secondary hyperparathyroidism,128,138 and hypervitaminosis A in the cat.55 Neurodiagnostic testing for patients with spinal cord disease may include vertebral column radiography, myelography, cross-sectional imaging such as computed tomography (CT) scan and magnetic resonance imaging (MRI), electrodiagnostic testing, cerebrospinal fluid analysis, and molecular and serologic testing. Although neurodiagnostic imaging and molecular diagnostics are detailed in Chapters 28 and 3, respectively, the utility of available neurodiagnostics is considered briefly here in the context of helping the clinician to discriminate among various medical disorders of the vertebral column and spinal cord. Cerebrospinal fluid analysis is a key component of the neurodiagnostic workup and an invaluable resource in both clinical and research settings. Although abnormalities in cerebrospinal fluid cytology and protein are relatively sensitive indicators of central nervous system disease, they are rarely specific for individual disease processes. On occasion, bacteria, fungi, protozoa, parasites, or tumor cells may be identified on microscopic examination of cerebrospinal fluid. However, this is extremely rare. The cerebrospinal fluid profile helps the clinician to narrow the differential diagnosis (Table 30-1) but must be interpreted in the context of case signalment, history, clinical signs, and neuroimaging. One must be especially cautious not to overinterpret the cerebrospinal fluid profile. For example, in confirmed cases of central nervous system neoplasia or inflammation, cerebrospinal fluid may be misleadingly normal. Conversely, although rare, a cerebrospinal fluid pleocytosis may be present in cases with minimal or no histopathologic evidence of parenchymal or meningeal inflammation.37 When these important caveats are considered, cerebrospinal fluid may provide valuable ancillary data for clinicians to make sound decisions in patients with spinal cord disease. Table • 30-1 Cerebrospinal Fluid Abnormalities Associated With Canine and Feline Spinal Cord Disorders Cerebrospinal fluid can be collected from the cerebellomedullary cistern or the lumbar cistern. Because the fluid flows predominantly in a rostrocaudal direction, it is more diagnostic and therefore preferable to collect it from a site caudal to the suspected lesion.132 Collection of cerebrospinal fluid requires the patient to be under general anesthesia with the site of collection clipped and aseptically prepared. No more than 1 mL of cerebrospinal fluid per 5 kg body weight (in dogs, cats, and horses) should be collected.40 The equipment required for sampling includes sterile plain collection tubes, 20 to 22 gauge, 40 to 90 mm (1.5 to 3.5 inch) spinal needles, and sterile surgical gloves. Small hypodermic needles for cerebrospinal fluid collection (22 or 25 gauge) may be useful in small dogs and cats and are safer if the clinician is inexperienced in assessing the depth of the relative cisterns from the skin surface. In such patients, an obvious “sensation” may not accompany penetration of the dura; a needle without a stylet, such as a hypodermic needle, will help the user identify the time of puncture of the dura with a “flash” of cerebrospinal fluid that simultaneously appears in the hub. A spinal needle may be used, with the stylet removed after the skin is pierced, but the larger needle makes the procedure cumbersome in a small dog. Generally, 0.75 to 2.0 mL fluid is sufficient for protein and cellular examinations.33 In our experience, most laboratories can comfortably assess protein levels, cytology, and cell counts when supplied with 0.5 mL of cerebrospinal fluid. A few drops should be saved in separate plain tubes for microbial culture and sensitivity if infection is suspected, and for virologic and immunologic studies, if needed. An EDTA or serum tube can be used if the sample is taken for polymerase chain reaction (PCR) analysis. Once collection is completed, the needle is gently removed from the site of collection, and if further fluid is required, the needle should be placed over the collection tube as it empties out its contents. Technically, lumbar collection is more difficult to perform than cerebellomedullary cistern collection and is more likely to result in iatrogenic blood contamination. The patient is positioned in lateral recumbency, with the pelvic limbs fully flexed. The appropriate intervertebral space is L5-L6 in dogs and L6-L7 in cats.40 At these spaces, the spinal cord has tapered into the conus medullaris and is surrounded by nerve roots or the cauda equina, both of which are much less likely than the cord itself to be damaged by needle insertion. The subarachnoid space (cistern) rarely extends to the lumbosacral junction in dogs,37 whereas collection sometimes may be made from the lumbosacral space in cats. The total number of cells present in cerebrospinal fluid typically is determined by using a cell counting chamber, such as a Fuchs-Rosenthal chamber. Ideally, counting should be performed within 30 minutes to 1 hour of cerebrospinal fluid collection, as cells may degrade in cerebrospinal fluid with low protein content. Refrigerating helps to minimize cellular degeneration. In the cerebrospinal fluid of normal dogs and cats, 0 to 5 WBCs × 106/L (WBCs/µL) is considered normal.40 A traumatic tap minimally affects the cell count. In cases with cerebrospinal fluid pleocytosis (>5 WBCs × 106/L [WBCs/µL]), the next step in the analysis is determination of the differential cell count via cytospin.40 After staining (e.g., Diff-Quik, Papanicolaou), the percentages of the different types of leukocytes should be counted, and the size and appearance of the cells should be evaluated. A close assessment for microorganisms, index of mitosis, and neoplasia should be completed. The utility of the cytospin is that the cytocentrifugation process concentrates all of the cells in a volume of 0.5 to 1.0 mL of cerebrospinal fluid. In the case of marked pleocytosis, 200 µL typically is sufficient for a differential cell count. If a cytospin is not available, a sedimentation chamber can provide reliable cell counts. Some laboratories prefer that protein (fetal calf serum or hetastarch) is added to cerebrospinal fluid samples to improve cytospin preparations.50 This is not critical when cerebrospinal fluid samples have a total protein elevation. In the dog and cat, normal total protein content evaluated from cerebrospinal fluid collected from the cerebellomedullary cistern typically is less than 250 mg/L (25 mg/dL), and it should be less than 450 mg/L (45 mg/dL) when collected from the lumbar cistern.40 Elevated total protein serves as a nonspecific indicator of central nervous system disease, and it may be caused by a damaged blood-brain barrier or by increased local (intrathecal) immunoglobulin (Ig)G production. Elevated cerebrospinal fluid total protein may be present in degenerative, anomalous, metabolic, neoplastic, infectious/inflammatory, traumatic, vascular, and toxic disorders. Infectious diseases should be considered when a cerebrospinal fluid pleocytosis is present in a dog or cat with a myelopathy. Bacterial and fungal culture of cerebrospinal fluid typically is reserved for cases in which the index of suspicion is relatively high for infectious disease, including the presence of systemic signs and blood count/biochemical abnormalities (e.g., fever, leukocytosis). In addition to cerebrospinal fluid culture, biologic samples such as urine and blood can be cultured to help pursue the diagnosis. Numerous sites should be cultured because of the low diagnostic yield of cerebrospinal fluid culture alone.103 Serologic testing should be performed when the index of suspicion is high for an infectious disease affecting the nervous system. Typical antibody titers evaluated in canine and feline central nervous system and peripheral nervous system diseases include Toxoplasma gondii, Neospora caninum, Ehrlichia spp., Rickettsia rickettsii, and Coccidioides immitis. Antigen testing, when available (e.g., Cryptococcus antigen testing), may circumvent problems associated with interpretation of antibody testing. Antigen testing, however, may be insensitive because it requires the presence of the organisms in the biologic sample under evaluation. The authors recommend evaluating antibody titers for regional infectious diseases and for pathogens to which animals may have been exposed during travel. Although antibody titers reflect direct exposure to the organism, a positive titer does not confirm active infection; titers should be evaluated in the context of the patient’s signalment, history, clinical signs, and neuroimaging results. The clinician should recall that IgM and IgG antibodies reflect acute and chronic infections, respectively. For example, a mildly elevated IgM titer (with no previous neurologic or systemic disease) may support an infectious cause, whereas a markedly elevated IgG titer simply may be indicative of previous exposure to a pathogen or vaccination, rather than active disease. Although rarely performed in clinical practice, serial antibody titers may be helpful for identifying causative agents; alternatively, antibody indices may be evaluated.3 An IgG antibody index may be calculated as a quotient by using the IgG and albumin content of cerebrospinal fluid and serum as follows to assess for intrathecal IgG synthesis: Measurement of IgA in cerebrospinal fluid and serum may be helpful, as combined elevation of cerebrospinal fluid and serum IgA levels is strongly suggestive of steroid-responsive meningitis-arteritis.137 Elevation of IgA in cerebrospinal fluid alone is less discriminatory and may indicate a primary (infectious/inflammatory disease) or a secondary immune response (e.g., neoplasia). Over the past decade, polymerase chain reaction (PCR) assays have been utilized routinely in veterinary medicine, thus providing a mechanism to detect and exponentially amplify small quantities of nucleic acids of an infectious agent (DNA or RNA) in biologic fluids or tissues. PCR applied to cerebrospinal fluid has revolutionized the diagnosis of human central nervous system infections and has similar potential in veterinary medicine. The sensitivity and specificity of PCR for the diagnosis of specific viral meningoencephalitis may be >95% and >99%, respectively, when cerebrospinal fluid is tested between 48 hours and 10 days after onset of neurologic signs.124,129 The PCR diagnosis of infectious meningoencephalitis in people allows for rapid implementation of targeted antimicrobial therapies and excellent survival rates compared with the situation in veterinary patients. In dogs and cats with meningoencephalitis, PCR of cerebrospinal fluid should be considered to test for regional pathogens. When combined with serologic testing, the chances of identifying a causative agent are maximized. Despite the limited soft tissue detail provided by CT scan, this modality may help to provide evidence of a noncompressive myelopathy, such as meningomyelitis or fibrocartilaginous embolic myelopathy, particularly when interpreted in the context of cerebrospinal fluid analysis. CT imaging characteristics for several inflammatory spinal diseases have been described102,131; however, CT results should be interpreted cautiously because of overlap in the imaging features of various spinal cord disorders. An important limitation of CT scan is that it produces a “beam hardening” artifact (due to preferential absorption of low-energy x-ray beams) that may obscure the clinician’s ability to interpret spinal cord lesions. The most common (nonneoplastic, nonanomalous) medical disorders of the vertebral column and spinal cord include degenerative myelopathy), meningo(encephalo)myelitis (MEM, including steroid-responsive meningitis-arteritis [SRMA], granulomatous meningoencephalomyelitis [GME], and infectious meningomyelitis), discospondylitis, and fibrocartilaginous embolic myelopathy. The clinician should consult Chapter 39 to review neoplastic disorders of the vertebral column and spinal cord, as well as a neurology textbook for more comprehensive details on rare medical disorders.37 The purpose of this chapter is to provide the background, clinical signs, etiopathogenesis, differential diagnosis, neurodiagnostics, treatment, and prognosis associated with degenerative myelopathy, steroid-responsive meningitis-arteritis, granulomatous meningoencephalomyelitis, infectious meningoencephalomyelitis, discospondylitis, and fibrocartilaginous embolic myelopathy. Background: Degenerative myelopathy (syn. degenerative radiculomyelopathy) is a neurodegenerative disorder that primarily affects the spinal cord of middle-aged versus older dogs, and rarely, older cats.4,37,90 The disorder is a diffuse axonopathy associated with necrosis primarily in the lateral and ventral funiculi of the thoracolumbar spinal cord segments (Figure 30-2).37 The axonopathy is accompanied by secondary demyelination and astrogliosis. Degenerative myelopathy is overrepresented in the German Shepherd Dog, the Pembroke Welsh Corgi, the Boxer, and the Rhodesian Ridgeback but also has been reported in the Siberian Husky, the Miniature Poodle, and the Chesapeake Bay Retriever.4,13,87 Although the overall prevalence of degenerative myelopathy in dogs has not been established, it may represent 1% to 5% of referral cases to neurology specialty practice.47 Clinical Signs: Degenerative myelopathy is a slowly progressive, nonpainful disorder with high morbidity and low mortality.* Neuroanatomic localization in dogs with degenerative myelopathy commonly occurs to the third thoracic through third lumbar (T3-L3) spinal cord segments, and the disease is typified by a progressive upper motor neuron paresis and proprioceptive ataxia in the pelvic limbs.29 Occasionally, mild asymmetry is noted in the pelvic limb signs. Segmental spinal reflexes typically are normal to exaggerated in the pelvic limbs. Despite the predominating upper motor neuron signs, in a low percentage of dogs (10% to 20%), unilateral or bilateral loss of the patellar reflexes may occur.37 Late in the disease, urinary and fecal incontinence, thoracic limb involvement, and rarely lower motor neuron signs may occur in the pelvic limbs.4,29,32,37 Humane euthanasia is a common endpoint because of the dog’s inability to support weight. Etiopathogenesis: Although the complete etiopathogenesis of degenerative myelopathy in dogs and cats has yet to be elucidated, it may involve a combination of genetic and environmental factors. Recent landmark studies have disclosed a genetic risk factor for degenerative myelopathy in dogs.5 Specifically, genome-wide association analysis of 38 Pembroke Welsh Corgi dogs disclosed a missense mutation (G-to-A nucleotide transition) in the superoxide dismutase (SOD1) gene. This mutation also has been demonstrated in German Shepherd Dogs, Boxers, Rhodesian Ridgebacks, and Chesapeake Bay Retrievers. SOD1 is an enzyme that converts superoxide radicals to hydrogen peroxide and oxygen, thus preventing superoxide radical damage in tissues. Some dogs that are homozygous for the SOD1 mutation develop progressive superoxide radical-induced axonal and myelin degeneration within the spinal cord. Therefore, dogs that are homozygous (two mutated alleles) for the SOD1 mutation are considered “at risk” for developing degenerative myelopathy. The distribution of lesions in canine degenerative myelopathy (including anti-SOD1 cytoplasmic inclusions) is very similar to that reported for the upper motor neuron–dominant (onset) form of amyotrophic lateral sclerosis (ALS) in human beings.42,69 As such, the discovery of the SOD1 missense mutation has provided the first spontaneous animal model of ALS in human beings. Because some dogs that are homozygous for the SOD1 mutation do not develop degenerative myelopathy (Coates, personal communication 2010, SJS), other modifying genes that influence phenotype and environmental factors are important considerations. It is interesting to note that feline leukemia viral antigen has been identified in the spinal cord lesions of cats with spinal cord pathology that is similar to degenerative myelopathy.23 Histopathologic similarities also exist among dogs and cats with degenerative myelopathy and among retrovirus-induced myelopathies in human beings, which are characterized by similar axonal and myelin degeneration and spinal cord lesion topography.82,88 The histopathologic similarity of canine degenerative myelopathy to human and feline retrovirus-induced myelopathies warrants investigation. Neurodiagnostics: Definitive diagnosis of degenerative myelopathy can be achieved only by histopathologic examination of the spinal cord (see Figure 30-2). A presumptive, antemortem diagnosis of degenerative myelopathy is made by ruling out other spinal cord disorders with spinal radiographs, MRI, and cerebrospinal fluid analysis, and by combining these neurodiagnostic tests with results of SOD1 mutation analysis. A dog with progressive T3-L3 signs that has normal neurodiagnostics and is homozygous for the SOD1 mutation is likely to have degenerative myelopathy, although SOD1 mutations are not 100% predictive of the disease.5 MRI should not reveal any structural abnormalities, and cerebrospinal fluid may be normal or may have elevated total protein (see Table 30-1). Concurrent abnormalities such as mildly compressive intervertebral discs may complicate the diagnosis. A 1- to 2-week antiinflammatory corticosteroid (0.5 to 1.0 mg/kg prednisone once daily) trial can be considered to help differentiate intervertebral disc disease from degenerative myelopathy, as the latter should not respond to corticosteroids. Treatment and Prognosis: At present, no therapy has been proven to alter the course of degenerative myelopathy. Most dogs succumb to the disease within months or a few years of diagnosis. Although some cases will progress slowly, others will progress more rapidly, and signs may be complicated by concurrent orthopaedic conditions such as hip dysplasia. Physical therapy may prolong survival time and generally is recommended to keep dogs with a presumptive diagnosis of degenerative myelopathy relatively active.72 Meningomyelitis is defined as inflammation of the spinal cord parenchyma and surrounding meninges. In a retrospective review of 220 dogs with inflammatory central nervous system disease, 41 animals had focal spinal cord involvement.136 Canine distemper virus and protozoa were the most commonly identified agents, and steroid-responsive meningitis-arteritis was the most frequently recognized noninfectious inflammatory cause.136 Rickettsiae,84 fungi,80a bacteria,39,103 helminthes,133,140 and granulomatous meningoencephalomyelitis constitute additional causes of meningomyelitis in dogs*; in many cases, however, the underlying pathology is not elucidated.60 Neurologic signs associated with meningoencephalomyelitis are variable and are related to the area of the spinal cord affected. Idiopathic or autoimmune meningomyelitides (e.g., steroid-responsive meningitis-arteritis, granulomatous meningoencephalomyelitis) predominate in the dog,60,134 whereas infectious meningoencephalomyelitis seems to be more common in the cat.85 It is critical to differentiate among the various idiopathic and infectious meningomyelitides and to differentiate meningoencephalomyelitis from surgical spinal cord disorders such as intervertebral disc disease. A description of the most common causes of canine and feline meningoencephalomyelitis follows. Steroid-Responsive Meningitis-Arteritis Background: Numerous and sometimes colorful synonyms (e.g., necrotizing vasculitis, polyarteritis, panarteritis, juvenile polyarteritis syndrome, beagle pain syndrome, corticosteroid-responsive meningitis, aseptic suppurative meningitis, steril eitrige meningitis) for steroid-responsive meningitis-arteritis (SRMA) are reflective of the clinical and histopathologic features associated with the syndrome. However, the diverse terminology for this disorder sometimes generates confusion among general practitioners and veterinary specialists. The name “steroid-responsive meningitis-arteritis” is well established in the veterinary literature and best describes the pathologic and clinical features of the disease, because it is a systemic immune disorder characterized by inflammatory lesions of the leptomeninges and associated arteries that typically is responsive to corticosteroids.37 The disorder may occur in any breed of dog, although Beagles, Boxers, Bernese Mountain Dogs, Weimaraners, and Nova Scotia Duck Tolling Retrievers are overrepresented. Age at onset is commonly between 6 and 18 months, with a range from 4 months to 7 years.28 Steroid-responsive meningitis-arteritis is an important differential for cervical spinal cord disease and is critical to differentiate from intervertebral disc disease and “Wobbler syndrome” in the aforementioned breeds. Clinical Signs: Steroid-responsive meningitis-arteritis is a sporadic disorder characterized by episodes of profound cervical hyperesthesia, depression, and pyrexia.37 Clinical signs result from combined meningitis and arteritis of leptomeningeal vessels (Figure 30-3). Arteritis also may involve the vessels of the heart, mediastinum, and thyroid glands.125 Occasionally, steroid-responsive meningitis-arteritis occurs concurrently with immune-mediated polyarthritis.139 Two forms of steroid-responsive meningitis-arteritis are known, including the “classic,” acute form and the chronic, protracted form. In acute steroid-responsive meningitis-arteritis, dogs most commonly present with hyperesthesia along the vertebral column, cervical rigidity, stiff gait, and fever.137 Affected animals often manifest a hunched posture with profound guarding of the head and neck, sometimes mimicking a cervical intervertebral disc protrusion. The condition may be so painful that any manipulation elicits a pain response. A second, more chronic form of steroid-responsive meningitis-arteritis may occur following relapses of acute disease and/or inadequate treatment.137 In this form of disease, meningeal fibrosis secondary to the inflammatory process may obstruct cerebrospinal fluid flow or occlude the vasculature, rarely causing secondary hydrocephalus or ischemia of the central nervous system parenchyma, respectively.125 Involvement of the motor and proprioceptive systems may lead to variable degrees of paresis and ataxia; other neurologic signs, such as a menace deficit, anisocoria, or vestibular signs, may occur with severe disease. Neurodiagnostics: Analysis of the cerebrospinal fluid in acute disease reveals marked polymorphonuclear pleocytosis, in addition to elevated protein and variable red blood cells.137 Red blood cells may be present in cerebrospinal fluid secondary to leakage from damaged vessels or contamination from peripheral blood. Typically, the cerebrospinal fluid neutrophils have no toxic changes; however, in severe cases, both banded and segmented neutrophils may be observed. Cerebrospinal fluid in the chronic form of steroid-responsive meningitis-arteritis may be variable, consisting predominantly of mononuclear cells or a mixed cell population with normal or mildly elevated total protein.137 Bacterial cultures are routinely negative. Radiographs of the cervical vertebral column are normal. CT scan or MRI may demonstrate contrast enhancement of the meninges.89 In some dogs, inflammation also affects the meninges of the brain and the choroid plexus.142 In both forms of steroid-responsive meningitis-arteritis, blood work may show neutrophilia with a left shift, an increased erythrocyte sedimentation rate, and an elevated alpha-2 globulin fraction.28 Most affected dogs have elevated IgA levels in both cerebrospinal fluid and serum—a finding that most likely is secondary to dysregulation of the immune system.45,136,137 Elevated serum and cerebrospinal fluid IgA levels help differentiate steroid-responsive meningitis-arteritis from other idiopathic and infectious canine meningoencephalitides; however, elevated IgA levels may be associated with primary or secondary inflammation. Elevated IgM and/or IgG in the cerebrospinal fluid also has been documented.136 More recently, acute phase proteins, including C-reactive protein and alpha-2 macroglobulin, have been shown to be elevated consistently in the serum of dogs with steroid-responsive meningitis-arteritis.9 However, elevation of acute phase proteins is not pathognomonic for the disorder, and other systemic inflammatory diseases, when present, should be included in the differential diagnosis. Once steroid-responsive meningitis-arteritis has been confirmed, elevated C-reactive protein serum concentrations, rather than repeated cerebrospinal fluid collection and analyses, may be used reliably to monitor response to therapy.9 These results were confirmed recently by Lowrie and others.83 Etiopathogenesis: The exact etiopathogenesis of steroid-responsive meningitis-arteritis is unknown.28 Activated T-cells have been demonstrated in dogs with steroid-responsive meningitis-arteritis, indicating potential contact with an antigenic stimulus; however, no bacterial or viral agents have been identified to date.135 A Th-2–mediated immune response is most likely to occur, on the basis of the presence of high CD4:CD8a ratios and high proportions of B-cells in peripheral blood and cerebrospinal fluid. A Th-2–mediated immune response is further supported by the expression of low levels of Th-1 response–related cytokines (interleukin [IL]-2, interferon [IFN]-γ) and upregulation of Th2 cytokines (IL-4) in blood and cerebrospinal fluid in dogs with the acute form of steroid-responsive meningitis-arteritis.114 This Th-2–mediated immune response leads to upregulation of the humoral immune response and excessive IgA production.114 Although autoantibodies have been demonstrated in steroid-responsive meningitis-arteritis, these antibodies are thought to be an epiphenomenon rather than the actual cause of the disease.112 Immunoglobulin deposition in blood vessel walls in steroid-responsive meningitis-arteritis lesions is rare; however, focal IgA deposition has been demonstrated in chronic cases.136 Chemotactic factors, including IL-8, have been identified in cerebrospinal fluid and correlate with IgA levels.20 The constant release of chemotactic factors may explain relapsing cases and an ensuing parenchymal form of disease that occurs when steroid therapy is discontinued.137 Dogs with relapses maintain high IgA levels and coinciding chemotactic activity. Upregulation of the integrin CD11a has been demonstrated in dogs with steroid-responsive meningitis-arteritis. Integrins are responsible for leukocyte recruitment to the central nervous system, and CD11A upregulation may be responsible for the neutrophilic pleocytosis typically associated with steroid-responsive meningitis-arteritis. It is interesting to note that the serum of dogs with steroid-responsive meningitis-arteritis induces CD11a upregulation on healthy neutrophils; soluble factors may be responsible for this phenomenon. It is hypothesized that CD11a expression is a key factor in neutrophil invasion into the subarachnoid space in steroid-responsive meningitis-arteritis.114 In addition, metalloproteinases, including matrix metalloproteinase (MMP)-2 and -9, have been shown to be upregulated in steroid-responsive meningitis-arteritis and likely disrupt the blood-brain barrier, contributing further to the neutrophilic pleocytosis.115 Beiner has suggested that oxidative stress contributes to the pathogenesis of steroid-responsive meningitis-arteritis and may lead to the protracted form of the disease.10 Corticosteroid therapy reduces oxidative stress and may prevent the transition from acute to chronic steroid-responsive meningitis-arteritis by preventing damage to the central nervous system vasculature or by suppressing the development of autoantigens. Treatment and Prognosis: The prognosis for steroid-responsive meningitis-arteritis is fair to good, especially in dogs with acute disease that are treated with early antiinflammatory and/or immunosuppressive therapy. Prednisolone or prednisone immunotherapy often is required for successful treatment outcomes with this disease. However, if initial signs are very mild and the neutrophilic pleocytosis is less than 200 cells/µL in the cerebrospinal fluid, nonsteroidal antiinflammatory drug therapy accompanied by careful patient monitoring may be sufficient in a subset of cases. Untreated dogs typically have a relapsing and remitting disease course. A study of 10 dogs with steroid-responsive meningitis-arteritis that received long-term treatment (4 to 20 months) showed that 8 of 10 dogs were free of clinical signs for up to 29 months after the treatment protocol was concluded.28 The following treatment regimen administered for a minimum of 6 months is recommended for typical cases of steroid-responsive meningitis-arteritis28: • Prednisolone or prednisone: 4 mg/kg/day, PO or IV initially. After 2 days, the dosage is reduced to 2 mg/kg PO daily for 1 to 2 weeks, followed by 1 mg/kg PO daily. • Dogs are reexamined every 4 to 6 weeks; cerebrospinal fluid analysis and hematology are repeated intermittently. • When clinical signs and cerebrospinal fluid are normal, the dose is reduced by half, until a dosage of 0.5 mg/kg PO every 48 to 72 hours is given. • Treatment is stopped about 6 months after clinical examination, cerebrospinal fluid, and blood profiles are normal.
Medical Conditions of the Nervous System
Neurologic Examination
Anomalies
Metabolic Disorders
Nutritional Diseases
Overview of Neurodiagnostics for Disorders of the Vertebral Column and Spinal Cord
Cerebrospinal Fluid Analysis
Cerebrospinal Fluid Collection
Cerebellomedullary Cistern Collection Technique
Lumbar Collection Technique
Cerebrospinal Fluid Cell Counts and Cytology
Cerebrospinal Fluid Total Protein
Microbial Culture
Serologic Testing and Polymerase Chain Reaction
Serology
Polymerase Chain Reaction
Neuroimaging (Myelography, Computed Tomography, and Magnetic Resonance Imaging)
Computed Tomography (CT)
Differential Diagnosis for Disorders of the Vertebral Column and Spinal Cord
Specific Disorders
Meningomyelitis
Idiopathic Meningomyelitis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Medical Conditions of the Nervous System
Only gold members can continue reading. Log In or Register to continue