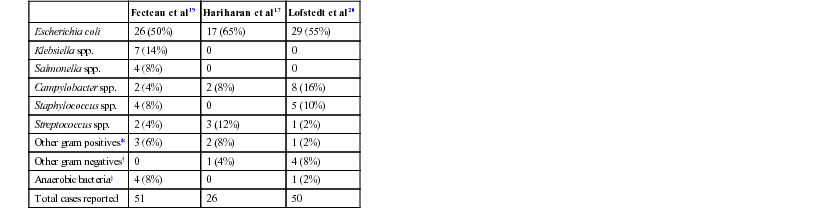
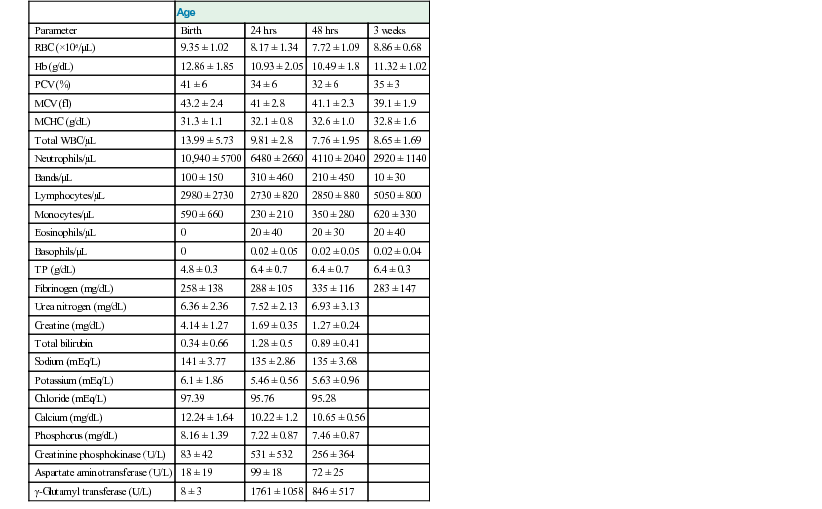
John K. House, Consulting Editor Geof W. Smith Sheila M. McGuirk Alison A. Gunn MatThew Izzo Geof W. Smith If weakness has been present since birth, in utero–acquired bacterial or viral infections, birth asphyxia and trauma, chronic placental problems, and congenital anomalies should be considered on the list of differential diagnoses. A number of congenital bacterial, fungal, and viral infections that cause abortions and stillbirths may result in the birth of a live but weak calf. In cattle, brucellosis, salmonellosis, leptospirosis, listeriosis, Escherichia coli, Corynebacterium spp., and Aspergillus spp. may cause placentitis and disease in the newborn. In sheep, in utero infection with chlamydia, Campylobacter, Coxiella, bluetongue virus, and border disease may cause disease in the newborn. Congenital viral infections of neonates are listed in Box 20-1. Clinical manifestations of fetal infections depend on the age of the fetus and the virulence and trophism of the infecting agent (see individual diseases). Neonatal calves with storage diseases primarily affecting the nervous system may appear reasonably normal for a short period after birth and then show progressive signs of neurologic dysfunction, including tremors, spasms, depression, recumbency, and coma. Differential diagnoses for weakness and depressed mentation after a period of apparently normal strength and mentation include sepsis, electrolyte and acid-base disturbances, hypoglycemia, and hypothermia. A complete history is obtained, including a detailed description of the delivery process, and complete physical and neurologic examinations are performed. Any signs of trauma, infection, or congenital malformations should be noted. Evaluation of hematologic data and immunoglobulin G (IgG) status, combined with historical and physical examination parameters, results in a high suspicion of sepsis. Blood glucose, blood gas, serum electrolyte concentrations, and passive transfer status should be determined promptly. Blood cultures and cerebrospinal fluid (CSF) analysis are useful for verifying central nervous system (CNS) involvement and targeting antimicrobial therapy. Neonatal septicemia is the third most common cause of calf mortality in the United States (behind diarrhea and respiratory disease) and occurs most commonly in calves associated with failure of passive transfer (FPT). The phagocytic and bacterial killing function of neutrophils (PMN) is a crucial component of the primary immune response against invading pathogens. Despite a larger number of neutrophils in the circulation of normal calves at birth, neutrophils from neonatal calves are functionally less effective than adult cells. Reduced Fc-receptor expression in neonatal PMNs may contribute to impaired phagocytosis and antibody-dependent cellular cytotoxicity.1–3 Depressed PMN bacterial killing4 may be related to reduced superoxide anion5 and myeloperoxidase-hydrogen peroxide-halide antibacterial activity.3 Adult-level superoxide activity in fetal PMNs suggests some of the deficits in neonatal PMN function may be a manifestation of perinatal PMN suppression.6 Calves have elevated cortisol levels for the first 10 days of life, which may contribute to depression of neutrophil function.7 Dexamethasone depresses neutrophil phagocytosis, antibody-dependent cellular cytotoxicity, and bacterial killing.8,9 Undefined serum factors also appear to be important for PMN function, as phagocytosis and bacterial killing by neonatal PMNs is similar to that of adult PMNs when bacteria are opsonized with adult serum but is reduced when bacteria are opsonized with neonatal serum.4 T-helper cells (CD4+) play a central role in humoral and cell-mediated immunologic memory. Lymphokines produced by CD4+ cells, interleukin-2 (IL-2), IL-4, and interferon-γ (IFN-γ) are essential components of antigen-specific immunity. Virtually all IL-4 and most IFN-γ produced by polyclonally activated adult human CD4+ cells is mediated by a subset of “functional memory cells.”10 Leukocyte production of IL-4 and IFN-γ is reduced in human neonates10 and IFN-γ in bovine neonates (Dr. David Van Metre, UC Davis, unpublished data), possibly reflecting their antigenically naive status. Depressed lymphocyte proliferation and IL-2 activity in the perinatal period correlates with elevated cortisol levels.7 Acquisition of humoral immune competence is age and antigen dependent.11 Neonates are capable of producing humoral immune responses to good immunogens (protein antigens) but may fail to respond to lesser immunogens (sugars and lipids). Calves less than 3 months of age vaccinated with modified live or killed salmonella vaccines produce an adult-type humoral immune response to salmonella protein antigens but do not respond to salmonella lipopolysaccharide.12 Ingestion of colostrum suppresses the humoral response to some antigens but not others.11 Adverse management and environmental conditions further compromise neonatal immunity. Cold stress depresses neutrophil chemotaxis, and vasoconstriction reduces delivery of leukocytes to peripheral tissues.13 Protein energy malnutrition in calves is associated with depressed lymphocyte IL-2 activity, lymphocyte proliferation, and humoral immune responses.7 Micronutrient deficiencies also depress immunity. Selenium, zinc, copper, and vitamin E deficiencies depress lymphocyte and phagocyte function.14,15 Inherited defects in immune function are sporadically observed in neonates and should be considered when recurrent or atypical infections are observed, such as bovine leukocyte adhesion deficiency in Holsteins. If invading bacteria are not quickly controlled by the immune system, they can establish focal infections in areas such as the joints, meninges, heart valves, or growth plates. Septicemia refers to a bacterial infection of the blood associated with adverse systemic signs. If not successfully treated, this can lead to multiple organ dysfunction, septic shock, and death. Meningitis is particularly difficult to treat successfully and therefore prevention of septicemia through good colostrum management is essential. Blood culture studies of debilitated calves indicate that gram-negative bacteria account for approximately 80% of bacterial isolates; E. coli is the most common bacteria isolated.16–18 In a study of 190 recumbent calves on a large calf-raising facility, 31% were determined to be bacteremic; E. coli accounted for 51% of the isolates; other gram-negatives, 25%; gram-negative anaerobes, 5.9%; gram-positive cocci, 11.8%; and gram-positive rods, 5.9%.16 Other common bacterial isolates include Salmonella, Campylobacter, Klebsiella, and different Staphylococcus species. A summary of bacterial pathogens found to be responsible for septicemia are listed in Table 20-1. Polymicrobial infections are common in septicemic calves (28%).18 TABLE 20-1 Bacterial Species Found to Be Associated with Neonatal Septicemia in Calves * Trueperella pyogenes (formerly Arcanobacterium pyogenes), Bacillus spp., Listeria monocytogenes. † Pseudomonas aeruginosa, Mannheimia haemolytica. ‡ Clostridial spp., Bacteroides spp., Prevotella spp. Calves lack a normal adult intestinal flora, and when born in a heavily contaminated environment, colonization of the GI tract with virulent bacteria may occur prior to the establishment of normal flora. Septicemia may also result from bacterial colonization of another site such as the umbilicus. Bacterial infection and the host inflammatory response should be differentiated. The immune response, in combination with the reticuloendothelial system, prevents the development of sepsis from an opportunistic or pathogenic invasion. However, this initiates an inflammatory cascade involving highly toxic mediators, which, if uncontrolled, will eventually lead to the systemic inflammatory response syndrome (SIRS) and subsequent multiple organ dysfunction syndrome (MODS). There is a balance between an appropriate, yet effective, immune response and an overzealous response to the bacteria or their toxins.21 The interaction between endotoxin (lipopolysaccharide) and the immune system triggers a complex inflammatory cascade involving cytokines and other inflammatory mediators. Initiation of the cascade results in the production of arachidonic acid metabolites, release of myocardial depressant factors, activation of the complement system, as well as the production and release of many other mediators of sepsis.21 The results include dehydration, tachycardia, pyrexia, leukopenia, hypotension, a decrease in systemic oxygen delivery and cardiac output, and generalized weakness. There is a severe and complex pulmonary vasculature response to endotoxin, leading to hypoxemia. Cattle are very sensitive to endotoxin, and even small doses can produce severe lung injury. Severe endotoxemia is frequently associated with death from respiratory failure in both calves and adult cattle. Therefore, nonsteroidal antiinflammatory drugs (NSAIDs) are critical in blocking the formation of reactive metabolites (e.g., thromboxanes) and restoring lung function. Neonatal septicemia should be seriously considered whenever there is multiple organ dysfunction, or when severe cardiac and/or respiratory signs are encountered. Classic septicemia is described as a condition affecting newborn calves between 2 and 8 days of age. The progression is rapid and most often fatal. Very early in the disease, the clinical signs are vague, nonspecific, and likely attributed to other disease. An alteration in mental status ranging from a mild depression to coma is commonly observed. Lack of suckling ability or enthusiasm toward nursing are early nonspecific clinical signs.21 Abnormal rectal temperature (fever or hypothermia) is not consistent; however, sustained tachycardia and eventually tachypnea develop. Hyperemia of the mucous membranes and scleral injection is frequently observed. Capillary fragility may initiate petechiation of the mucous membranes. Eventually, hypotension and clinical signs that are associated with poor cardiac output (slow capillary refill, diminished peripheral pulse, cold extremities, and decreased urine output) become prominent. Dehydration usually develops. Diarrhea is not present in all cases but is common in the terminal stages of septicemia. Early clinical signs of septicemia can be subtle and nonspecific. Sepsis scoring models have been developed for calves but lack good sensitivity and specificity.19,20 The most useful parameters for predicting septicemia in calves as determined by these studies include toxic changes in neutrophils, FPT, presence of a focal infection, or elevated serum creatinine concentrations. Ultimately, the presence of clinical signs including lethargy, pyrexia, diarrhea, tachypnea, polyarthritis, uveitis, omphalitis, and meningitis, along with documented presence of FPT, should make the clinician highly suspicious of septicemia. If multiple clinical signs are present, the likelihood of disease is increased. However, the definitive diagnosis of septicemia can only be based on a blood culture. The jugular vein should be clipped and scrubbed prior to sampling. An amount of 10 mL of whole blood is drawn using a sterile syringe and needle, and the blood is placed into a blood culture bottle using a new sterile needle. Taking two samples (from blood collected 1 hour apart) is sometimes recommended to increase the chances of isolating the bacteria and to facilitate the interpretation of results if opportunistic or contaminating bacteria are found in one sample.21 The bottle is then submitted to the laboratory for culture and susceptibility testing. If the bottle is not submitted immediately, it should be stored at room temperature or at 37° C. A negative blood culture must be interpreted with caution since many factors may interfere with bacterial isolation from a blood culture. Cultures of other body fluids such as joint, cerebrospinal, peritoneal, or pleural fluids may be helpful for bacteriologic diagnosis whenever the blood culture is negative. Some laboratory findings may increase the suspicion of septicemia. Hematologic abnormalities of septicemia vary with the severity of the disease. Abnormal neutrophil count (neutrophilia and neutropenia) and increased immature forms (bands) are frequently seen. Fibrinogen concentration is often elevated. Thrombocytopenia may be present in severe cases. One study of suspected septic shock in calves found that 8 out of 12 calves had at least three abnormal coagulation parameters, most commonly activated partial thromboplastin time (APTT) and prothrombin time (PT).22 Hypoglycemia, or less often hyperglycemia, may be observed. A metabolic acidosis is also frequently present in septic calves. Lactic acidosis occurs as the disease progresses. Some calves will develop respiratory disease (respiratory distress syndrome) or pneumonia and will suffer from hypoxemia and/or hypoventilation. Neonatal septicemia is a severe disease with high mortality. The primary goals of treatment are to (1) control the infection, (2) modulate the inflammatory response, and (3) support the animal during the disease. Treatment is based on selecting an appropriate antimicrobial drug and dosage, antiinflammatory therapy, and supportive therapy (including fluids). Differences between neonatal and adult animals in drug effects generally can be attributed to differences in drug distribution, metabolism, or excretion. Some general characteristics of the neonatal period include better absorption of drugs from the gastrointestinal tract, less drug binding to plasma proteins, increased apparent volume of distribution of drugs that are distributed in the extracellular fluid volume, increased permeability of the blood-brain barrier, and slower elimination (i.e., longer half-life) of many drugs. In general terms, antimicrobials have longer elimination times in neonates (<2 weeks of age) than adults and as such larger doses are administered with a longer dosage interval to achieve similar peak and trough antimicrobial concentrations. Antimicrobial therapy should begin as soon as possible in order to minimize the bacteremia. The choice and the dosage of antibiotic depend on several factors, and personal preference, along with past experience, is often important. It is generally accepted that the intravenous route is preferable whenever possible and that broad-spectrum drugs are preferred. Appropriate antimicrobial choices include third- or fourth-generation cephalosporins (ceftiofur 5 to 10 mg/kg/day IM or IV), sodium ampicillin (10 to 20 mg/kg tid IV), and florfenicol, although even when given intravenously at the extralabel dosage of 20 mg/kg bid, it only exceeds the MIC90 value in plasma for 1 hour.23 Combinations of drugs can be used to broaden the spectrum of activity (such as ampicillin-ceftiofur or ampicillin-trimethoprim-sulfonamide). Fluoroquinolones can also be effective in countries where their use is permitted. Once the pathogen has been identified and the susceptibility pattern determined (on the individual or on the farm), the most appropriate antibiotic can be chosen. As just described, the inflammatory response if uncontrolled often causes severe clinical signs. Therefore, NSAID therapy should be considered for ancillary treatment. Possible side effects of aggressive NSAID treatment include abomasal ulcers and renal toxicity, particularly in dehydrated animals. The duration of NSAID treatment is often limited to 2 to 3 days because of their potential toxicity. NSAIDs should not be continued for any longer than they are considered essential for survival. Supportive treatments for septicemic calves include correction of secondary problems, administration of fluids, plasma transfusion, oral or parenteral nutrition, and oxygen administration. Problems that must be addressed include hypovolemia, hypoglobulinemia, hypoglycemia, metabolic acidosis, electrolyte abnormalities, and hypoxia. IV fluids (or oral as a compromise on the farm) should be administered. Dextrose (5%) combined with normal saline (0.9%) should be administered at a rate of at least 50 mL/kg/day. Nutrition is important and septic calves either nurse reluctantly or are inappetant; as a result, they do not ingest an adequate amount of milk. If the animal refuses to nurse, tube feeding should be used to ensure that the calf ingests at least 10% to 15% of its body weight of milk per 24 hours. The feeding schedule may involve several (three to five) feedings per day and should be gradually increased in amount to prevent abdominal distention. If the gastrointestinal system does not tolerate the tube feeding regimen, parenteral nutrition should be considered. There is usually no need for total parenteral nutrition since most animals will continue to nurse or tolerate tube feeding up to 5% of their body weight. Multiple cases of septicemia occurring on the same farm calls for careful inspection of the environment and management practices. Clusters of septicemia could be related to specific risk factors. As an example, feeding heavily contaminated colostrum could be the origin of the septicemia on some farms.22,24 Prevention through excellent cleanliness and management practices is the most effective means of controlling and preventing neonatal sepsis. Bacterial meningitis is an important complication associated with neonatal septicemia in calves. Exactly how bacteria get into the meninges is poorly understood. Specific mechanisms may include the development of a sustained and high-grade bacteremia in the highly perfused dural venous system and choroid plexuses, adherence of fimbriae from some strains of E. coli, or the phagocytosis of the pathogens by circulating monocytes and endocytosis through the microvascular endothelial cells.21 Once they gain access to the CNS, bacteria survive and proliferate well in the poorly defended CSF. Complement is essentially nonexistent in CSF, which, when combined with low numbers of specific antibodies, leads to inadequate opsonization of meningeal pathogens. The sequelae of meningitis are associated with the release of cytokines and the direct effects of bacterial invasion. Bacteria may release endotoxin, leading to inflammatory infiltrates that cause thromboses of the arachnoidal or subependymal veins. Congestion or hemorrhagic infarction may follow with subsequent necrosis of nerve cells. Inflammatory changes in the subarachnoid space may affect the choroid plexus, decreasing the absorption of fluid and potentially creating hypertensive hydrocephalus. Although bacterial meningitis may occur as a primary entity, it more commonly is a result of generalized septicemia in neonates with FPT. Agents that cause meningitis are the same agents that cause septicemia, most commonly the gram-negative enteric bacteria such as E. coli, Enterobacter spp., and Salmonella spp. In a review of 32 cases of meningitis in calves, the clinical signs of CNS disturbance observed were lethargy, recumbency, anorexia, loss of suckle reflex, coma, opisthotonos, convulsions, tremor, and hyperesthesia.25 Leukocytosis and a left shift were evident in 11 of 15 calves (73%). Concurrent metabolic problems were common and included hyperkalemia, respiratory acidosis, hypernatremia, hyponatremia, hypomagnesemia, and hypoglycemia. Analysis of CSF revealed pleocytosis, xanthochromia, turbidity, and high total protein concentration. Cytologically, neutrophils predominated in the CSF in calves with acute disease. Mononuclear cells dominated in calves with chronic disease. Microscopically, bacteria were evident in 10 of 22 (45%) of the antemortem CSF samples, and bacteria were isolated from slightly more than half (11 of 19). All of the calves in this review died.25 Calves with meningitis are often presented because they have lost their suckle reflex and appear lethargic. Previous treatment for diarrhea is common. Fever is often present unless NSAIDs have been given or if the animal is in an extremely cold environment. The calves may have an extended head and neck, and attempts to flex or reposition the neck can result in a tonic extension and thrashing of the limbs. Calves with meningitis almost always have abnormal mentation. As the disease progresses, profound depression develops and eventually the animal becomes comatose and nonresponsive or may develop seizures. Presumptive diagnosis of bacterial meningitis is based on demonstration of FPT, presence of a septic focus such as omphalophlebitis or septic arthritis, and the presence of abnormal neurologic signs. However, the definitive diagnosis is based on an abnormal CSF analysis. Collection of CSF from the lumbosacral space is easy and safe in calves. For collection of fluid from the lumbosacral space, a 20-gauge, 1- to 2-inch needle with a clear hub may be used. A change in resistance is felt when the needle penetrates the dural membranes, and CSF appears in the plastic hub as soon as the subarachnoid space is entered. Approximately 5 to 10 mL of fluid may be removed safely. Urinary reagent strips can be used to rapidly obtain general information about the fluid. If blood is detected, the sample should be spun down after the cytologic examination. Red blood cells contaminating the sample will settle, and the supernatant should be colorless. If hemorrhage occurred before the procedure, the sample remains xanthochromic (yellow). Glucose should be present in “trace” or “positive” (+) amounts in the normal sample. Negative values in the adult suggest severe meningitis, but in the neonate they may also be caused by profound hypoglycemia. CSF analysis is most useful in determining the presence of septic meningitis. A summary of CSF findings from calves with meningitis is presented in Table 20-2. Generally an elevation in total protein and white blood cell concentration are strongly suggestive of meningitis, combined with a high percentage of the cells being neutrophils. The ratio of CSF to plasma glucose concentration is less than 1 in animals with bacterial meningitis because of bacterial metabolism of glucose in the CSF. Xanthochromia (yellow color) is inconsistent. Free or intracellular bacteria may be observed in some cases. An elevated albumin quotient suggests increased blood-brain permeability and can be seen in both hypoxic-ischemic brain injury and meningitis, but an elevated IgG index indicates increased intrathecal IgG production and is more compatible with a diagnosis of meningitis. TABLE 20-2 Cerebrospinal Fluid (CSF) Findings in Calves with Meningitis When treating calves with meningitis in a hospital environment, the mortality rate is high; however, aggressive early treatment can be successful. The economics and welfare implications of treating commercial calves in a field setting are questionable. Empiric antimicrobial therapy for meningitis in neonatal calves should include a gram-negative and gram-positive spectrum. Antibiotics enter the CSF predominantly via passive diffusion down a concentration gradient. The major determinant of CSF penetration is lipid solubility. Lipophilic agents diffuse via transcellular pathways; peak concentrations in CSF occur relatively rapidly, and entry into CSF is affected minimally by the presence of inflammation. In contrast, hydrophilic agents enter the CSF through paracellular pathways; their transport depends on the opening of tight junctions, and peak concentrations are relatively delayed.26 Table 20-3 lists CSF-to-blood concentration ratios (penetration) derived from multiple species for a handful of antimicrobial drugs available for use in cattle. TABLE 20-3 Cerebrospinal Fluid–to–Blood Concentration Ratios (Penetration) of Antibiotics Available for Treatment of Meningitis in Calves23,26 * The list is not conclusive, reflecting the paucity of available data. CSF, Cerebrospinal fluid. In a CSF pharmacokinetic study of florfenicol in calves, the maximum concentration of florfenicol attained in CSF was 4.67 ± 1.51 µg/mL following a single intravenous dose of 20 mg/kg.23 The levels remained above the minimum inhibitory concentration (MIC) for Histophilus somni (formerly Hemphilus somnus) over a 20-hour period. This concentration is below the MIC90 for E. coli. Bacteriocidal antibiotics are proposed to be more effective for treatment of meningitis in humans, and it is recommended that the concentration of antibiotic in the CSF should be maintained at 10 times the MIC of the target pathogen.26 Unfortunately, owing to the lack of CSF pharmacokinetic data in cattle, antimicrobial treatment of meningitis is an inexact science. Antiinflammatory therapy and nursing care should be instituted as described previously for neonatal septicemia. Convulsions in calves can be treated with diazepam (0.1 to 0.2 mg/kg IV). This can be repeated every 30 minutes until convulsions are controlled. Profound weakness associated with metabolic acidosis or “strong ion” acidosis is commonly observed in calves with diarrhea and sporadically in kids (“floppy kid syndrome”) and calves without other clinical signs of disease.27,28 This acidosis is often associated with an increased concentration of D-lactate (the anion of D-lactic acid) resulting from bacterial fermentation of carbohydrates in the gastrointestinal tract of milk-fed calves. Clinical signs of impaired CNS function including ataxia and coma in sick calves have been attributed at least partially to the increased D-lactate concentrations.29,30 Assessment of weak neonatal ruminants for the presence of metabolic acidosis is a critical part of the physical examination. Correction of the acidosis by intravenous administration of bicarbonate produces a rapid recovery. An improvement in mentation and strength should be observed within 12 hours; persistent depression is likely to reflect incomplete correction of acidosis, sepsis, hypoglycemia, hypernatremia, or hyponatremia. Hypoglycemia is a common sequela to withdrawal of milk for more than 48 hours, especially in cold weather. Affected calves are weak or recumbent but appear to be normally hydrated or minimally dehydrated.31 They are often emaciated and can occasionally have neurologic signs including facial twitches, convulsions, opisthotonos, and coma. They will respond to a bolus of 50% dextrose or infusion of 5% glucose, but often this response is temporary, especially in calves with severe malabsorptive disease. It is important to rapidly restore adequate energy intake to ensure resolution of these cases. Starvation and hypothermia resulting from mismothering are common causes of weakness in neonatal lambs. Similarly, weakness, poor body condition, and increased susceptibility to infectious diseases are observed with protein-calorie malnutrition induced by feeding poor-quality or incorrectly mixed milk replacers.32 Hypoglycemia is also frequently seen in calves with neonatal septicemia, as discussed previously. Hyponatremia occurs when loss of isotonic fluid through the gastrointestinal tract is replaced by free water or hypotonic solutions. The latter often occurs when too much water is added when making up an oral electrolyte solution. Hyponatremia may also occur when isotonic oral electrolyte solutions are administered to calves with compromised sodium absorption capacity. This may be a result of severe pathologic changes or an inadequate level of agents that facilitate sodium cotransport within the oral electrolyte solution. Hyponatremia results in a fluid shift from the extracellular space to the intracellular compartment along the osmotic gradient, and the resultant swelling of the cells can result in neurologic disturbances, depression, disorientation, and even convulsions.33 Hyponatremia should be considered in calves with serum sodium less than 132 mmol/L; calves with serum sodium less than 120 mmol/L have severe hyponatremia. The goal of therapy is to restore serum sodium levels to greater than 125 mmol/L over the first 6 hours and then to restore to normal levels over 24 hours.33 In hypovolemic calves the initial treatment should be achieved using normal saline, and in normovolemic calves hypertonic saline should be used for the initial treatment, since the administration of large fluid volumes will exacerbate cerebral edema. If the calves are also suspected to be acidotic, this should be corrected with sodium bicarbonate solutions of appropriate tonicity. The amount of sodium required in the first 6 hours to raise the sodium level to 125 mmol/L can be calculated as follows33: Calves should then be maintained on a sodium-containing isotonic fluid, such as normal saline or lactated Ringer, and treated with oral electrolyte solution as appropriate. The sodium level should be monitored frequently in the first 24 hours because of unknown losses through the gastrointestinal tract as well as unknown kidney function in a severely dehydrated patient. Hypernatremia is defined as a serum sodium concentration greater than 152 mmol/L (although only levels greater than 170 mmol/L have been associated with nervous dysfunction).34 Hypernatremia in food animals is generally caused by one of the following: (1) excessive loss of free water (e.g., water deprivation, heat stress), (2) iatrogenic administration of IV crystalloid solutions to animals that do not have access to water, and (3) excessive intake of sodium without an adequate volume of free water. This last cause is by far the most common syndrome in calves. Most cases result from mixing oral electrolyte solutions improperly, or in some circumstances, hypernatremia has been seen with very high osmolality milk replacers. During hypernatremic states, water will follow concentration gradients and move into the relatively hyperosmolar CSF and plasma. This results in cellular dehydration and brain shrinkage (neurons shrink when the water moves out). Neuronal cells will increase their intracellular osmolality in an attempt to minimize the water efflux; however, this adaptation can only compensate for mild increases in sodium concentrations. Severe hypernatremia leads to neurologic disease in calves. Early clinical signs of hypernatremia include lethargy and depression, which, without blood work, cannot be differentiated from many other possible diseases such as acidemia, dehydration, hypoglycemia, or hypothermia. More advanced clinical signs of hypernatremia include twitching of facial muscles, muscle rigidity, tremors, and myoclonus.34,35 Calves will demonstrate seizure and/or coma activity near death. Prognosis in these calves is often guarded to poor even with aggressive treatment. Treating hypernatremia is very difficult. If the sodium concentration is lowered too quickly, water follows a concentration gradient and moves into neurons, resulting in cerebral edema. When sodium concentrations are higher than 160 mEq/L, it is very easy to produce cerebral edema with any isotonic fluid, even those that contain sodium, because of the large difference between the calf’s extracellular fluid sodium concentration and the sodium concentration in the fluids. Therefore, it becomes necessary to add supplemental sodium to standard fluid types. The fluids must be formulated so that the sodium concentration is equal to or slightly lower than the calf’s sodium concentration.34 The calf’s sodium concentration should be reassessed at least one to two times per day and fluids reformulated. The goal would be to return the sodium concentration to normal over a period of several days, decreasing it by 3 to 4 mEq/L/day.35 This approach is expensive (requiring frequent blood work) and difficult and is not always successful. Some calves will still develop cerebral edema even with careful fluid therapy. For example, if a calf has a sodium concentration of 177 mEq/L, the goal would be to give fluids with a sodium concentration somewhere in this range (175 to 177 mEq/L). Since isotonic (0.9%) saline has a sodium concentration of 154 mEq/L, the clinician needs to add 22 to 23 mEq/L of additional sodium to the liter of fluids. Hypertonic saline (7.2%) contains sodium at a concentration of 1.2 mEq/mL. This would be equal to 19 mL of hypertonic saline per liter of isotonic saline (1.2 × 19 = 23). After about 12 hours of fluid therapy, it is recommended to reassess the calf’s sodium concentration and re-formulate fluids. Hypertonic saline could also be added to isotonic sodium bicarbonate (which has a sodium concentration of 156 mEq/L) if the calf had a metabolic acidosis in addition to hypernatremia. Although not always successful, a report of treating several hypernatremic calves with 5% dextrose either alone or in combination with sodium bicarbonate has been published.36 In this study calves were given 2 to 4 L of 5% dextrose at a slow drip (about 500 mL/h) and had serum sodium concentrations decreased much faster than is usually recommended. However, the four calves in the case report were treated successfully. Although this approach may not always work and could easily result in cerebral edema, it may be practical for times when the clinician does not have the ability to formulate fluids or monitor serum sodium concentrations frequently. If the calf’s neurologic signs get significantly worse after fluid therapy has started, cerebral edema should be suspected. Treating cerebral edema is difficult but imperative to prevent further brain damage and/or death. Corticosteroids are the easiest choice normally; however, they are only marginally effective in treating moderate to severe cases of cerebral edema. Mannitol (25%) at 1 g/kg given IV over 30 minutes or an oral solution of glycerin diluted 1 : 1 with water has also been recommended for severe cases of cerebral edema but can be more difficult to obtain.34 Primary neuromuscular or musculoskeletal disease should be considered when weakness is not associated with depressed mentation. Weakness associated with micronutrient deficiencies results from myodegeneration (white muscle disease, selenium, and vitamin E) or demyelination (copper, enzootic ataxia). If weakness is detected in one or more limbs immediately after birth, peripheral nerve and muscle damage associated with birth trauma should be ruled out (see Box 20-1). Femoral nerve paralysis may be observed in calves following a “hip lock” dystocia.37 A condition resembling congenital myasthenia gravis has also been described in Brahman calves.38 Nutritional myodegeneration associated with selenium and or vitamin E deficiencies may produce localized (dysphagia) or generalized paresis. Neonatal small ruminants appear to be particularly susceptible. Affected lambs may be unable to rise; others can stand but may be unable to nurse because they are unable to raise their heads. Diagnosis is based on clinical signs, increased serum creatine kinase concentration, and reduced whole blood glutathione peroxidase and/or selenium concentrations (see Chapter 42). Vitamin E deficiency is observed when pregnant ewes are fed stored forage low in vitamin E; the clinical signs in affected lambs are identical to selenium deficiency, but selenium status is adequate. Since vitamin E is labile, serum should be harvested quickly after blood collection, frozen, wrapped in aluminum foil, and sent express mail on ice. Paraplegia and tetraplegia are commonly associated with spinal cord compression. Compression of the spinal cord in neonates most commonly results from vertebral body malformations, osteomyelitis, or fractures. Generally vertebral body malformations occur sporadically; genetic, nutritional, and environmental factors have been implicated.39,40 In older calves, underlying metabolic bone disease (copper, vitamin D, or phosphorous deficiency) may increase the propensity for fractures to occur. Osteomyelitis and vertebral body abscess may be a sequel to bacteremia following neonatal septicemia or pneumonia.41 The frequent isolation of Trueperella pyogenes from vertebral body abscesses in ruminants suggests that chronic respiratory infections is more frequently the source in these species.42,43 Vertebral body abscesses in lambs are occasionally a sequel to infected docking wounds. Leukocytosis and hyperfibrinogenemia are commonly observed in neonates with vertebral body abscesses. In most instances vertebral abscesses do not infiltrate the pachymeninges, so the CSF either is normal or has a mild elevation of protein and/or a mild pleocytosis.41,42 Differential diagnoses for paresis in goat kids include caprine arthritis encephalitis virus (CAEV) and enzootic ataxia. Enzootic ataxia is also common in lambs. Progressive ataxia and paresis or paralysis is a feature of both diseases. There are two forms of enzootic ataxia (swayback): the neonatal and the delayed types. In the neonatal condition animals are affected at birth in the delayed type; signs of incoordination appear at 14 to 30 days of age.44 Most affected kids are afebrile, bright, and alert, and they will continue to eat if it is physically possible. Enzootic ataxia is associated with low liver copper content, and occasionally, low serum copper concentration.45 It has been proposed that reduction in the activity of the copper-dependent enzyme cytochrome oxidase impairs phospholipid synthesis and subsequently myelin production. Microcytic anemia and increased fragility of bones may be observed in more chronic cases.46 The copper, molybdenum, and sulfur content of the maternal diet should be evaluated and adjustments made for copper deficiency or molybdenum or sulfur excess (see Chapter 32). Goat kids with the neurologic form of CAEV will have mild to moderate fevers and evidence of cerebral involvement. Cerebral signs commonly identified include depression, head tilt, torticollis, and circling.47 Evidence for CAEV would include CSF pleocytosis and increased CSF protein and a positive CAEV (AGID) or enzyme- linked immunosorbent assay (ELISA) test. Both the neurologic form of CAEV and enzootic ataxia carry a poor prognosis. A complete neurologic examination is an important component of the work-up of the weak neonate. In particular, it should be noted if the weakness is accompanied by signs of depression and diffuse cerebral disease. Strength is preserved if ataxia is caused by cerebellar disease. Limb reflexes should be tested to establish whether components of the spinal reflex pathways are involved in the disease process (sensory nerve, lower motor neuron, neuromuscular junction, muscle). Animals with other types of spinal cord disease (e.g., trauma, vertebral malformations, enzootic ataxia) may also show weakness and ataxia yet appear clinically to have normal cerebral function. Virtually any severe systemic disease such as generalized infection can cause both profound depression and weakness in a neonate without the presence of actual brain pathology. Intermittent signs of severe weakness and depression may be caused by the narcolepsy-cataplexy syndrome. Sheila M. McGuirk Respiratory conditions of the neonatal ruminant can be attributed to upper airway problems, pulmonary pathology, CNS dysfunction, cardiac disease, metabolic derangements, or other nonpulmonary disorders (Box 20-2). A complete examination of the neonate is essential when clinical signs are indicative of a respiratory condition. When a single individual is involved, observation from a distance, recording of vital signs, and a physical examination guide the clinician to the selection of appropriate diagnostic tests to establish a cause, make a diagnosis, and evaluate the prognosis. For respiratory conditions that affect a flock or herd of animals, the examination of affected individuals remains an essential component of the diagnostic work-up, but evaluation of records, the environment (housing and other animals), nutrition, colostrum, treatment, and vaccination protocols is also needed. From a distance, mentation, posture, respiratory rate, breathing effort, characters of nasal and ocular discharge, and spontaneous coughing can be evaluated. Abnormal postures exhibited by patients with respiratory conditions may be recumbency, open-mouth breathing, abnormal extension of the head and neck, head tilt, or reluctance to lie down. Abnormal breathing characteristics include prolongation of the inspiratory or expiratory phase of respiration, an expiratory abdominal press or snap, marked tachypnea, frequent spontaneous coughing, stridor, or inspiratory or expiratory noises. Practitioners must assess airflow from each nostril and evaluate ocular, oral, pharyngeal, and vaginal mucous membrane characteristics, including temperature, color, moisture, and capillary refill time. The hard and soft palate should be evaluated. Animals with cleft palate may have milk run from the nose and are prone to developing aspiration pneumonia. Gray or cyanotic mucous membranes are associated with severe hypoxia (i.e., Pao < 35 to 40 mm Hg), and/or circulatory collapse as seen with hypotensive, endotoxic, or hypovolemic shock. Cardiac and pulmonary causes of cyanosis must be distinguished. Cyanosis from congenital cardiac disease of the neonatal ruminant frequently reflects right-to-left shunting of blood that may be from a tetralogy of Fallot, transposition of the aorta, great vessel anomalies, or Eisenmenger complex. Absence of cyanosis is not a reliable indicator of adequacy of oxygenation in the neonate, since the partial pressure of oxygen may reach very low levels (<35 to 40 mm Hg) before cyanosis is observed. Auscultation of the trachea, thorax, and occasionally the nasal passage are essential elements of the respiratory examination. Tracheal compression should not induce multiple coughs. Lung sounds of neonates are typically easier to hear than those of adults; however, lung sounds do not always correlate well with the severity of respiratory condition that is present. Interpretation of the intensity of thoracic breath sounds can be validated by comparison to tracheal breath sounds. The intensity of tracheal breath sounds is gradually attenuated as the stethoscope is moved from the trachea to the lung hilus, again to the anteroventral thorax, and finally to the caudal thorax in normal animals. Loud tracheal breath sounds that are not attenuated over the thoracic area may be indicative of atelectasis, lung consolidation, or cardiac enlargement. Added breath sounds such as crackles or wheezes should be noted but may not be heard without augmenting the depth and frequency of the breathing effort with the use of a rebreathing bag. Cardiac auscultation will determine heart rate, rhythm, and the presence of murmurs. Abnormal radiation of heart sounds caudal to the fifth intercostal space or dorsal to the level of the scapulohumeral joint on either side of the thorax should be noted. Thoracic percussion may be used to identify the presence of thoracic fluid, cranioventral consolidation, or atelectasis in neonatal ruminants. Heart and respiratory rates vary with activity, excitement, and feeding. Tachypnea can be attributed to elevated temperature humidity index, fever, thermoregulation dysfunction, excitement, pain, stress, respiratory disease, hypoxia (cardiac disease, pulmonary disease, shock), dehydration, shock, toxemia, or metabolic acidosis. Periodic apnea and abnormally slow respirations are often the result of metabolic disturbances (hypoglycemia, hypocalcemia), hypothermia, prematurity, or hypoxia-induced suppression of the respiratory center. Abnormal posture of the head and neck are exhibited by neonates with respiratory difficulty, particularly upper airway obstruction, but meningoencephalitis, otitis media, hypernatremia, polioencephalomalacia, congenital defects of the CNS, and lasalocid toxicity should be considered. Conditions causing occlusion of the upper airway, such as choanal atresia, necrotic laryngitis, pharyngeal swelling, or edema, often induce pronounced inspiratory dyspnea, stridor, and/or open-mouth breathing. Expiratory stridor and increased and prolonged expiratory effort are usually associated with lower airway disease. A malodorous breath may be present with necrotic pharyngeal injuries, necrotic laryngitis, cleft palate, or aspiration pneumonia. Inspiratory stridor is often a feature of extra-thoracic airway obstruction and increased abdominal effort on expiration an indication of pulmonary disease causing reduced lung compliance. Lung disease in the newborn is usually diffuse and is the result of infection acquired in utero or postpartum and/or lung atelectasis associated with immaturity, recumbency, or surfactant dysfunction. Signs of lung disease include increased work of breathing characterized by nostril flare, rib retractions, and increased abdominal effort. A cough and nasal discharge—salient features of respiratory disease in older neonates—are infrequent findings in newborns with lung disease. Animals with few or no audible thoracic abnormalities may have severe respiratory disease. A complete blood count, serum chemistry profile, electrolyte concentrations, arterial or venous blood gas concentrations, and a test of immune transfer status provide useful information to the clinician concerned about a respiratory condition in a neonatal ruminant. Normal values for neonatal calves are presented in Table 20-4. TABLE 20-4 Normal Hematology and Serum Chemistry Reference Values (for Neonatal Calves)48,49 Knowing whether there is anemia, evidence of infection, endotoxemia, organ dysfunction, metabolic abnormalities, hypercarbia, hypoxemia, or FPT of immunity may distinguish upper airway problems from lower airway disease or pulmonary disease from a nonpulmonary cause of respiratory difficulty. Arterial blood gas concentrations provide a useful measure of respiratory function. Optimal sites for collection of arterial blood samples from neonatal calves are the brachial artery proximomedial to the elbow50 or the caudal auricular artery.51 Normal arterial blood gas values for neonates of different postnatal and gestational ages are presented in Table 20-5. TABLE 20-5 Normal Arterial Blood Gas Values for Calves
Manifestations and Management of Disease in Neonatal Ruminants

Weakness and/or Depressed Mentation
Septicemia
Fecteau et al19
Hariharan et al17
Lofstedt et al20
Escherichia coli
26 (50%)
17 (65%)
29 (55%)
Klebsiella spp.
7 (14%)
0
0
Salmonella spp.
4 (8%)
0
0
Campylobacter spp.
2 (4%)
2 (8%)
8 (16%)
Staphylococcus spp.
4 (8%)
0
5 (10%)
Streptococcus spp.
2 (4%)
3 (12%)
1 (2%)
Other gram positives*
3 (6%)
2 (8%)
1 (2%)
Other gram negatives†
0
1 (4%)
4 (8%)
Anaerobic bacteria‡
4 (8%)
0
1 (2%)
Total cases reported
51
26
50
Meningitis
Normal
Green et al25
Scott et al25a
Specific gravity
<1.007
NR
1.011
Total protein
<0.4 g/L
3.07 g/L
3.2 g/L
White blood cell concentration
<10 cells/µL
4004 cells/µL
2400 cells/µL
Percent lymphocytes
>80%-90%
NR
Range of 1%-17%
Percent neutrophils
<10%-15%
73%
Range of 78%-100%
Culture positive
6 of 9 cases
11 of 19 cases

Concentration CSF/Concentration Serum (%)
Antimicrobial*
Human
Animals
Ampicillin
13-14
8-12
Florfenicol
46 (calves)
Gentamicin
0-30
21-25
Penicillin
5-10
5-6
Trimethoprim-sulfamethoxazole
<41
35-39
Metabolic Acidosis
Hypoglycemia
Hyponatremia
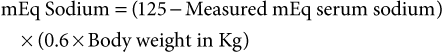
Hypernatremia
Neuromuscular and Musculoskeletal Disease
Respiratory Distress
Examination
Respiratory Condition Diagnostic Testing
Age
Parameter
Birth
24 hrs
48 hrs
3 weeks
RBC (×106/µL)
9.35 ± 1.02
8.17 ± 1.34
7.72 ± 1.09
8.86 ± 0.68
Hb (g/dL)
12.86 ± 1.85
10.93 ± 2.05
10.49 ± 1.8
11.32 ± 1.02
PCV (%)
41 ± 6
34 ± 6
32 ± 6
35 ± 3
MCV (fl)
43.2 ± 2.4
41 ± 2.8
41.1 ± 2.3
39.1 ± 1.9
MCHC (g/dL)
31.3 ± 1.1
32.1 ± 0.8
32.6 ± 1.0
32.8 ± 1.6
Total WBC/µL
13.99 ± 5.73
9.81 ± 2.8
7.76 ± 1.95
8.65 ± 1.69
Neutrophils/µL
10,940 ± 5700
6480 ± 2660
4110 ± 2040
2920 ± 1140
Bands/µL
100 ± 150
310 ± 460
210 ± 450
10 ± 30
Lymphocytes/µL
2980 ± 2730
2730 ± 820
2850 ± 880
5050 ± 800
Monocytes/µL
590 ± 660
230 ± 210
350 ± 280
620 ± 330
Eosinophils/µL
0
20 ± 40
20 ± 30
20 ± 40
Basophils/µL
0
0.02 ± 0.05
0.02 ± 0.05
0.02 ± 0.04
TP (g/dL)
4.8 ± 0.3
6.4 ± 0.7
6.4 ± 0.7
6.4 ± 0.3
Fibrinogen (mg/dL)
258 ± 138
288 ± 105
335 ± 116
283 ± 147
Urea nitrogen (mg/dL)
6.36 ± 2.36
7.52 ± 2.13
6.93 ± 3.13
Creatine (mg/dL)
4.14 ± 1.27
1.69 ± 0.35
1.27 ± 0.24
Total bilirubin
0.34 ± 0.66
1.28 ± 0.5
0.89 ± 0.41
Sodium (mEq/L)
141 ± 3.77
135 ± 2.86
135 ± 3.68
Potassium (mEq/L)
6.1 ± 1.86
5.46 ± 0.56
5.63 ± 0.96
Chloride (mEq/L)
97.39
95.76
95.28
Calcium (mg/dL)
12.24 ± 1.64
10.22 ± 1.2
10.65 ± 0.56
Phosphorus (mg/dL)
8.16 ± 1.39
7.22 ± 0.87
7.46 ± 0.87
Creatinine phosphokinase (U/L)
83 ± 42
531 ± 532
256 ± 364
Aspartate aminotransferase (U/L)
18 ± 19
99 ± 18
72 ± 25
γ-Glutamyl transferase (U/L)
8 ± 3
1761 ± 1058
846 ± 517
Age Group
O2 (mm Hg)
CO2 (mm Hg)
pH
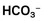 (mEq/L)
(mEq/L)
1 hour
58.43 ± 11.61
50.40 ± 5.27
7.30 ± 0.05
23.52 ± 2.78
4 hours
62.30 ± 9.27
47.92 ± 3.97
7.34 ± 0.03
24.49 ± 2.35
12 hours
67.23 ± 9.32
45.36 ± 3.97
7.38 ± 0.03
25.74 ± 2.37
24 hours
70.53 ± 11.47
44.04 ± 3.45
7.40 ± 0.03
26.44 ± 1.87
48 hours
63.85 ± 10.82
45.25 ± 3.69
7.42 ± 0.01
27.98 ± 1.91 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Manifestations and Management of Disease in Neonatal Ruminants
Chapter 20