Chapter 22 Managing Fluid and Electrolyte Disorders in Renal Failure
Fluid treatment
Fluid therapy for hospitalized patients
Although oliguria or anuria are the classic manifestation of acute kidney injury (AKI), AKI may present with polyuria, which frequently portends a less severe renal injury.4,64 AKI may also be a subtle increase in creatinine (>50% of baseline) or urine volume inappropriate for the volume of fluid administered. In this early stage of injury, attempts to lessen further renal damage are warranted. Patients with chronic kidney disease (CKD) may present in a decompensated uremic crisis, which may represent an acute kidney injury superimposed on chronic disease.
Assessing Hydration
The key feature to an appropriate fluid plan is accurate determination of hydration status. Blood volume can be measured using indicator dilution techniques, radioactive tracers, bioimpedance spectroscopy, or other methods.54 Unfortunately, readily available accurate measurement of blood volume is not feasible in general practice settings.
Despite a lack of precise objective data, there are many ways to estimate hydration. A deficit of the extravascular fluid compartment (interstitial and intracellular) causes dehydration. A severe deficit may decrease the intravascular compartment, leading to poor perfusion. Dehydration of less than approximately 5% is difficult to detect clinically. A 5% to 6% deficit leads to sticky mucous membranes. Six to eight percent dehydration causes dry mucous membranes and decreased skin elasticity. By 8% to 10% dehydration, the eyes may be sunken, and over 12% dehydration, corneas are dry, mentation is dull, and perfusion is impaired.28 Overhydration may manifest as wet mucous membranes, increased skin elasticity (heavy or gelatinous), shivering, nausea, vomiting, restlessness, serous nasal discharge, chemosis, tachypnea, cough, dyspnea, pulmonary crackles and edema, pleural effusion, ascites, diarrhea, or subcutaneous edema (especially hock joints and intermandibular space).13,36
Central venous pressure (CVP) measurement through a centrally placed intravenous catheter may provide information about intravascular filling. A volume depleted animal will have a CVP less than 0 cm H20. A CVP over 10 cm H20 is consistent with volume overload or right-sided congestive heart failure.62 However, pleural effusion falsely elevates the CVP.22 An accurate body weight recorded before an illness is an invaluable aid to assessing hydration. Body weight should be measured two to four times a day on the same scale to monitor fluid balance. A sick animal may lose up to 0.5% to 1% of body weight per day due to anorexia; changes in excess of this amount are due to changes in fluid status.10 An increase in blood pressure may indicate a gain of fluid; conversely, a decrease in blood pressure may indicate a net loss of fluid. Because of the high percentage of patients with hypertension (80% of dogs with severe acute uremia and 20% to 30% of dogs and cats with CKD), the trend rather than the absolute value is of more utility in assessing changes in hydration status.13,26,59 Similarly, changes in trends for PCV and total solids may reflect changes in volume, in the absence of bleeding or blood transfusion. Because each parameter is impacted by aspects beyond hydration status, these factors must be viewed in aggregate.
Type of Fluid
Colloidal solutions (i.e., hydroxyethyl starch, 6% dextran) may be appropriate if hypoalbuminemia is present. Hypoalbuminemia may be present with protein-losing nephropathy, diseases associated with vasculitis, or severe gastrointestinal losses or bleeding. The recommended dose is 20 mL/kg/day, and may be used to replace the insensible portion when using the “ins-and-outs” method (see later discussion). Higher doses may be associated with coagulopathy. Despite initial concerns that colloidal solutions may cause acute kidney injury (specifically, osmotic injury), there is no evidence that colloids are directly nephrotoxic.46 An alternative to synthetic colloids is human albumin, but this product carries a risk of anaphylaxis.11,21 Canine and feline albumin have recently become commercially available and can be used for colloidal support.
Volume and Rate
Some patients may present in hypovolemic shock, which is manifest as dull mentation, hypotension (systolic blood pressure <80 mm Hg), poor perfusion of the periphery (cold extremities, pale/gray mucous membranes with slow capillary refill time), hypothermia, or tachycardia.62 Immediate correction of shock is necessary to prevent further and irreversible organ damage. The standard dose of crystalloids is 60 to 90 mL/kg for dogs and 45 to 60 mL/kg for cats, of which one fourth is given over 5 to 15 minutes.41 If hemodynamic parameters do not improve sufficiently with the first one fourth dose, a second dose should be given. Resuscitation efforts are continued until the patient is hemodynamically sound. If the patient remains hypotensive and there are concerns about volume overload, central venous pressure monitoring may be helpful. A CVP less than 0 cm H2O indicates hypovolemia, whereas a CVP more than 10 cm H2O is a contraindication to further fluid therapy. A 10 to 15 mL/kg bolus of crystalloid or 3 to 5 mL/kg of colloid will not change the CVP in hypovolemic patients, but will transiently increase the CVP by 2 to 4 cm H2O in the euvolemic patient, and cause a rise of more than 4 cm H2O in the hypervolemic patient.62 Adequate resuscitation as assessed by achievement of identifiable goals decreases renal morbidity as compared with using standard resuscitation doses in people.30
Not withstanding the conventional wisdom that fluid therapy is cornerstone of treatment of kidney failure, evidence of harm from volume overload is mounting. Rapid restoration of renal perfusion may decrease renal damage, but there is no evidence that fluid therapy will reverse established renal injury.44,60 Patients with volume overload (>10%) had decreased survival and impaired renal recovery.6–8 In fact, one study in adult humans found that a 1 L positive fluid balance in 24 hours was associated with a 20% increase in mortality.42
Critically ill patients frequently have increased capillary leakiness, leading to tissue edema as a consequence of aggressive fluid therapy.44 Tissue edema impairs oxygen delivery and metabolite diffusion, distorts tissue architecture, and impairs capillary blood flow and lymphatic drainage.44 The adverse effects of tissue edema may be more predominant in encapsulated organs, such as the kidneys and liver, as the increased tissue volume increases interstitial pressure and decreases organ blood flow. Cardiac dysfunction caused by increased preload and myocardial edema further impairs tissue oxygen delivery and may impair renal recovery.44,53 The lungs are perhaps the most sensitive to volume excess, and the development of pulmonary edema is a common life-threatening condition in oliguric dogs and cats on fluid therapy.
Converting Oliguria to Nonoliguria
A decrease in urine production may be due to hemodynamic, intrinsic renal, or postrenal causes. An appropriate renal response to inadequate renal perfusion from hypovolemia or hypotension includes fluid retention with a concomitant decrease in urine volume. Before determining whether oliguria is pathologic or physiologic, renal perfusion should be optimized by ensuring adequate hydration. A volume of fluid equal to 3% to 5% of body weight should be administered to patients that appear normally hydrated because dehydration of less than 5% cannot be detected clinically. In patients that are volume overexpanded, this fluid administration is not necessary. Healthy kidneys can autoregulate renal blood flow at perfusion pressures between 80 to 180 mm Hg, but renal perfusion may be more linear in damaged kidneys.10,12 The mean arterial pressure should be maintained above 60 to 80 mm Hg, or the systolic pressure above 80 to 100 mm Hg when measured by Doppler technology. Apparent anuria due to obstruction of the urinary tract or leakage into the peritoneal, retroperitoneal, or subcutaneous tissues should be excluded before determining that a lack of urine is due to intrinsic renal damage.
Various values have been used to define oliguria, including less than 0.25 mL/kg/hr, less than 0.5 mL/kg/hr, and less than 1 to 2 mL/kg/hr.13 In a hydrated, well-perfused patient, less than 1.0 mL/kg/hr can be considered absolute oliguria, and urine production between 1 and 2 mL/kg/hr in a patient on fluid therapy is considered relative oliguria.10,13 Anuria is defined as essentially no urine production.13 Urine volume above 2 mL/kg/hr is generally considered polyuria.
If pathologic oliguria or anuria persists despite correcting hemodynamic parameters, most clinicians attempt to convert oliguria to nonoliguria using diuretics. There is no evidence that diuretics improve the outcome of AKI, and some surmise that the ability to respond to diuretics is a marker of less severe renal injury associated with a better prognosis. In people, an increase in urine output with diuretic use delays referral for dialysis, perhaps inappropriately.38 However, in veterinary medicine where dialysis is not as readily available to control fluid status, an increase in urine output from diuretic use may allow an increase in the volume of other medications or nutrition, and may be justified even without improvement in renal function.
Mannitol is an osmotic diuretic that causes extracellular volume expansion, which can improve GFR and inhibit sodium reabsorption in the kidney by inhibiting renin. Mannitol also increases tubular flow, which may relieve intratubular obstruction from casts and debris. Mannitol decreases vascular resistance and cellular swelling; increases renal blood flow, the GFR, and solute excretion; protects from vascular congestion and RBC aggregation; scavenges free radicals; induces intrarenal prostaglandin production and vasodilatation; and induces atrial natriuretic peptide release5,10,13,20 Mannitol may blunt the influx of calcium into mitochondria in sublethally injured renal cells, thus decreasing the risk of sublethal injury progressing to lethal damage. Despite the theoretical advantages, no randomized studies have shown a better clinical response with the use of mannitol and volume expansion than with volume expansion alone in people or healthy cats.20,37
Mannitol is administered as a slow intravenous bolus of 0.25 to 1.0 g/kg. If urine production increases, mannitol may be administered as a constant rate infusion (CRI) of 1 to 2 mg/kg/min IV or 0.25 to 0.5 g/kg q 4 to 6 hr.13 Doses in excess of 2 to 4 g/kg/day may cause ARF. Mannitol should not be given to patients that are dehydrated because it will further exacerbate intracellular dehydration. Conversely, it is also contraindicated if overhydration is present, and may worsen pulmonary edema.
Hypertonic dextrose can be used as an osmotic diuretic, if mannitol is not available. A total daily dose of 22 to 66 mL/kg of a 20% dextrose solution should cause hyperglycemia and glucosuria.47
Loop diuretics such as furosemide can increase urine flow without increasing the GFR.14,20,37,40,61 Despite the increase in urine output, loop diuretics do not improve outcome, suggesting that those who respond have less severe renal failure, resulting in a better outcome for a recovery independent of drug therapy.14,20,40,55,61 For example, in one human study, patients that could be converted from oliguric to nonoliguric renal failure had better APACHE scores (a disease severity scoring system used for people in ICU settings) and higher creatinine clearance before treatment, suggesting that they had less severe renal injury.55 Due to the perception that there is a low complication rate associated with the loop diuretics, they are often used despite lack of proven benefit. Loop diuretics inhibit the Na+-K+-2Cl− pump in the luminal cell membrane of the loop of Henle, decreasing transcellular sodium transport. Basal Na+, K+-ATPase activity becomes unnecessary and the medullary oxygen consumption decreases, which is hypothesized to protect the kidney from further injury.25,55 The amount of structural damage to the thick ascending limb of the loop of Henle is subsequently decreased in isolated perfused kidneys.25 Loop diuretics also have renal vasodilatory effects.45 Despite the theoretical reasons to use loop diuretics, one retrospective study in people showed an increased risk of death or failure of renal recovery in the furosemide treatment group. Potential reasons for this finding include a detrimental effect of the drug, delay in recognizing the severity of renal failure with subsequent delay in starting dialysis, or preferential use of loop diuretics in patients with a more severe course of disease.14,38 Loop diuretics may make fluid management easier in people, without changing the outcome.55 In animals, loop diuretics may play a larger role in management because dialysis is not universally available. Established indications for use of furosemide in veterinary medicine include treatment of overhydration or hyperkalemia.13 Furosemide should not be given to patients with aminoglycoside-induced ARF.10
An increase in urine output should be apparent 20 to 60 minutes after an intravenous dose of furosemide of 2 to 6 mg/kg. Ototoxicity has been reported at high doses in people, and doses of 10 to 50 mg/kg may cause adverse effects in animals (apathy and anorexia in cats; hypotension, apathy, and staggering in dogs).10 If there is no response to high doses of furosemide, therapy should be discontinued. If a response does occur, this dose can be administered every 6 to 8 hours. A continuous rate infusion gives a more sustained diuresis with a lower cumulative dose compared with bolus infusion.14 In people, the time to maximal effect with a CRI without a loading dose is 3 hours, and 1 hour with a loading dose. The dose in people is usually 1 to 9 mg/hr (about 0.01 to 0.15 mg/kg/hr) with some reports using doses as high as 0.75 mg/kg/hr.34 In normal dogs, 0.66 mg/kg/hr resulted in diuresis, and doses of 0.25 to 1 mg/kg/hr have been used in dogs and cats with naturally occurring renal failure.1,2,13 Because electrolyte and fluid balance disorders can develop rapidly if a brisk diuresis ensues, frequent monitoring is necessary.
Dopamine has been shown to make some human oliguric patients nonoliguric, but it does not increase the GFR or improve the outcome in people.20,23,40,50 Because of lack of efficacy and side effects associated with dopamine, it is no longer recommended for treatment of oliguric renal failure, except for pressor control.13,56 Selective dopamine agonists may have better efficacy and fewer adverse effects compared to dopamine. There are two dopaminergic receptors, DA-1 and DA-2. Fenoldopam is a selective DA-1 receptor agonist, and as such, it selectively increases cortical and medullary blood flow, sodium excretion, and urine output while maintaining the GFR in people. It does not have DA-2 or α or β adrenergic activity, so it does not cause vasoconstriction, tachycardia, or arrhythmias as seen with dopamine.20,45 Although no studies have shown a benefit, studies with this drug in people are encouraging and larger clinical trials are needed.45 Although some studies in dogs show an improvement in the GFR with fenoldopam, the GFR may decrease in the first few hours after administration.24,39,57
Calcium channel antagonists have been used to decrease damage after renal transplantation.35 Calcium channel antagonists presumptively reverse renal vasoconstriction by causing predominantly preglomerular vasodilatation, inhibit vasoconstriction induced by tubuloglomerular feedback mechanisms, and cause natriuresis independent of the GFR.35 Although the results of one study using diltiazem in addition to standard care in dogs with AKI from leptospirosis were not statistically significant, there was a trend toward increased urine output and more complete resolution of azotemia.35 Whether this will prove to be a beneficial therapy requires further study.
Atrial natriuretic peptide (ANP) increases tubular excretion of salt and water, and stimulates afferent arteriolar dilation and efferent arteriolar constriction, increasing the GFR. Although ANP reduced the severity of experimental ARF from ischemic but not nephrotoxic causes, it has not been effective in clinical trials thus far.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


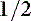 strength LRS + 2.5% dextrose).
strength LRS + 2.5% dextrose).