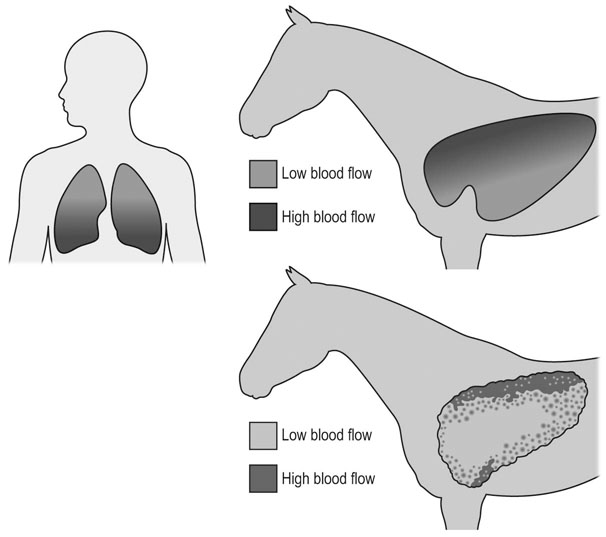
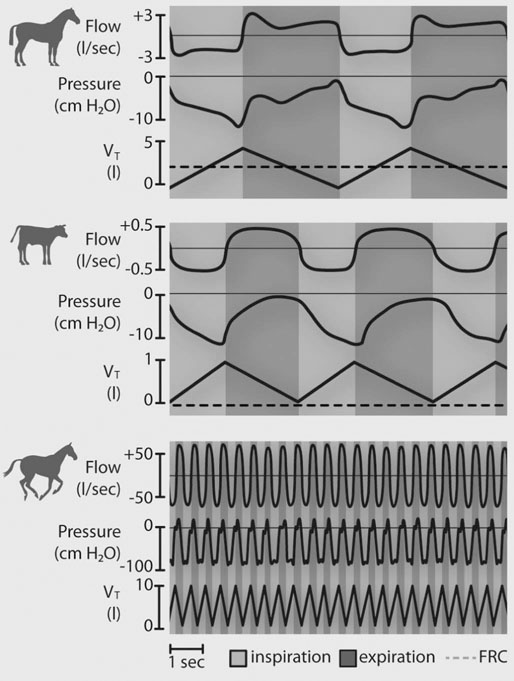
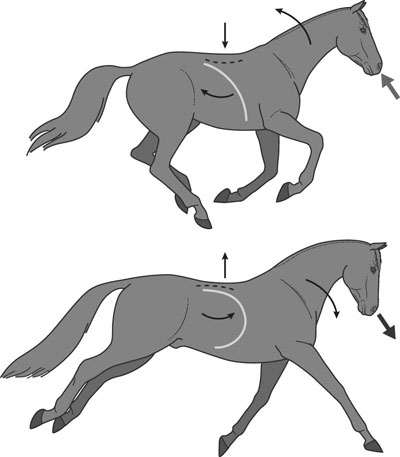
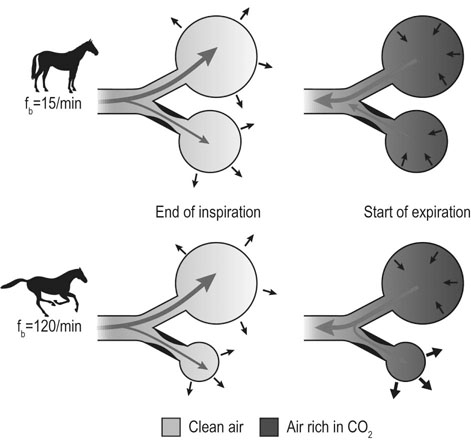
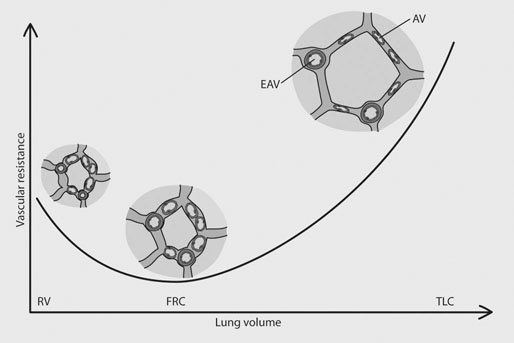
In most species, the healthy respiratory system is able to ensure adequate ventilation, as evidenced by PaO2 and PaCO2 values that differ minimally from those at rest, during exercise of any intensity. This is not the situation with horses which develop hypoxemia at relatively low exercise intensities (i.e. 60% VO2max) and hypercapnia at high exercise intensity. 1 Given the numerous and substantial respiratory adjustments that occur in this species during exercise, this impairment of gas exchange can be at first glance surprising. Nevertheless, some anatomic and physiologic peculiarities of this species combined with the huge metabolic demands at the level of the working muscles can explain this relative inability of the lung to maintain adequate gas exchange during heavy exercise. From a clinical point of view, the importance of a healthy respiratory system in the performance horse is highlighted by several retrospective studies of racing horses or endurance horses with exercise intolerance. All these papers reported a major impact of lower airway disease on exercise capability.2–4 Air is delivered into the deep part of the lung through conducting airways that carry ambient air from the nose to the alveoli during inspiration. Airflow is reversed during expiration. Gas exchange occurs at the level of the alveolar duct and the alveoli, which are lined by an extensive capillary network. The intrathoracic trachea, the bronchi and the bronchioles constitute the lower airway. The trachea is divided into right and left mainstem bronchi that enter the hilum of each lung. Each bronchus in turn divides into lobar, segmental and sub-segmental bronchi which end in bronchioles. The terminal bronchioles are poorly developed respiratory bronchioles, or open directly into alveolar ducts. Epithelium of the tracheobronchial tree is tall columnar pseudostratified interspersed with ciliated cells as well serous and mucus-producing goblet cells.5 Short ciliated cells and non-ciliated (Clara) cells constitute the epithelium of the bronchioles. Lastly, alveoli are mainly covered by type I pneumocytes, with type II pneumocytes producing surfactant (Fig. 27.1). The latter is essential to maintain the stability of alveoli, especially at low lung volumes. The epithelium in the large airway is covered by a double mucus layer, this system forming the mucociliary escalator which is an efficient system to clear the airway from the airborne particles deposited by impaction in the deep part of the lung (Fig. 27.1). Unlike the lung in most other animals, the horse lung is not divided into distinct lobes. There are fibrous connecting septa comprising an intricate mesh of interconnecting elastic and collagenous tissue fibers6 that divide the lung into lobules. This separation limits the collateral ventilation between lung regions – the ventilation of adjacent lobules through other pathways than the bronchial tree such as anastomosing bronchioles – but increases pulmonary interdependence (the mechanical interaction between adjacent regions) which enhances synchronization of ventilation of the different regions of the lung. Because the collateral pathways have a rather high resistance to airflow, they are probably of limited usefulness whatever the respiratory frequency. 6 On the other hand, the interdependence between adjacent lung areas can induce abnormal stresses on some lobules. Indeed, with increased airflow resistance, decreased compliance, or both, the tissues are stretched and compressed by the surrounding lung parts.7 It has been suggested that exercise-induced pulmonary hemorrhage could be a consequence of this pulmonary overstretching.8 The lung is perfused with arterial blood from the pulmonary and bronchial circulations. Blood flow distribution is mainly influenced by gravity and shows consequently a vertical gradient according to the relative magnitude of pulmonary arterial, venous and alveolar pressures in humans.9 The ventral regions in the lungs of humans receive more perfusion per unit lung volume than the dorsal regions, and blood flow in the lung can be divided into three or four zones (Fig. 27.2). It has been long thought that the same was true for horses, but recent work indicates that at rest gravity is a minor factor in determining blood flow distribution, despite the large hydrostatic gradient imposed by the height of the lung. Blood flow is preferentially distributed in the dorsal regions of the lung (thus opposite to gravity),10 a phenomenon which is exacerbated by exercise.12 The exercise-induced changes of blood distribution are described later in the text. There is a large heterogeneity of blood distribution within the same isogravitational plane, showing that pulmonary blood flow depends on factors other than the pressure gradient between pulmonary arteries and veins, such as the vessel’s length and resistance in a given area.11 The bronchial circulation is a high pressure system. It comes from a single vessel, branching from the aorta and receiving, at rest, 2% of the cardiac output. It subsequently divides into a right and left bronchial artery at the hilar region of the lung. It provides nutritional blood flow to the lymphatic, vascular and airway components as well as to the pleura. Bronchial circulation seems to play a role in warming and humidifying the inspired air, as suggested by the increase of its blood flow during exercise.13 Bronchial circulation could be involved in local inflammatory processes, as suggested by its proliferation occurring in horses with lesions of exercise-induced pulmonary hemorrhage (EIPH).14 When pulmonary perfusion is locally impaired, the bronchial circulation can proliferate and at the level of the terminal bronchioles, pulmonary and bronchial circulations can anastomose. Most of these anastomoses occur between capillaries and veins rather than with arteries.15 They maintain some blood flow throughout the lung, thus contributing partly to gas exchange. Such proliferations also can occur during pulmonary inflammation. Because they are extensive in the dorso-caudal regions of the lungs, which are the sections that sometimes bleed during exercise, this neovascularisation has been related to the occurrence of EIPH.14 The thorax provides rather rigid protection for the intrathoracic organs. The diaphragm, which separates the thorax from the abdomen, and the external intercostal muscles are the main inspiratory muscles. In horses, the serratus ventralis has an important role in assisting the inspiratory effort of the diaphragm, both at rest16 and during exercise.17 The mechanical equilibrium of the equine respiratory system (including thorax, lung parenchyma and muscles) lies at the midpoint of the tidal volume, contrary to other species in which the equilibrium point occurs at functional residual capacity. Consequently, in horses, both inspiration and expiration have a physiological biphasic pattern, with an initial passive and a second active component for each phase18 (Fig. 27.3). The intercostal muscles are activated in the second part of inspiration (for the external ones) and expiration (for the internal ones). 16 The transversalis muscle is the principal expiratory muscle. Lastly, the abdominal muscles (external and oblique abdominis, transverse and rectus abdominis, transverse thoracics) participate also to the active phase of expiration. When they contract, they increase the abdominal pressure, forcing the relaxed diaphragm forward and reducing the thoracic volume. However, during exercise, the biphasic shape of the respiratory airflow is no longer visible because of the very high respiratory frequency (Fig. 27.3). The activity of the respiratory muscles can be assessed either directly by electromyographic measurement or indirectly, by assessment of the respiratory muscle perfusion. Electromyographic studies of respiratory muscle recruitment have been performed in healthy horses at rest,1,16 and during exercise.19 Electrical activation of the diaphragm, as well as its mechanical output, increases linearly with exercise intensity.19 Ventilatory muscle perfusion has been studied in ponies as an indicator of muscle activity.17,20 The ventilatory muscles receive 10% of the cardiac output at rest and 15% during maximal exercise. During exercise, despite the activation of the external intercostal muscles (which should move the ribs forward and outwards), the thoracic circumference decreases slightly.21 This is probably related to the large negative pressures occurring in the thorax when the diaphragm contracts. The role of external intercostal muscle contraction consequently could be a stiffening of the thoracic structure to help to resist its physical deformation, rather than a genuine increase of the thoracic volume. Also, during exercise the abdominal circumference does not change either, which leads to the conclusion that in this circumstance the increase in tidal volume is due to an elongation of the thoracoabdominal segment.22 A study was recently performed to assess the effect of girth tension on respiration and performance ability in horses during supramaximal exercise. Although there was a decrease of the time to fatigue associated with high girth tensions, this was not due to alteration of respiratory mechanics or troubles in gas exchange. The authors suggested that this loss in performance could be related to inspiratory muscles working at suboptimal lengths due to thoracic compression or compression of musculature around the chest.23 The smooth muscles of the vessels and the bronchioles are innervated by sympathetic, parasympathetic and non-adrenergic non-cholinergic nervous systems. In the healthy horse, the lack of changes in pulmonary resistance after β-adrenergic stimulation or muscarinic blockade24 reflect that there is no smooth muscle tone in the bronchi of healthy horses at rest. The main factors involved in gas exchange are: • ventilation (i.e. how air gets to the alveoli) • perfusion (i.e. removal of gas from the lungs by the blood) • ventilation–perfusion ratio (i.e. how matching of air and blood distribution in the lung influences the gas exchange) • diffusion (i.e. how gas gets across the air–blood barrier) • gas transport (i.e. how gases are moved from lungs to the tissues) • mechanics of breathing (i.e. how the lungs are moved) and • control of breathing (i.e. how gas exchange is adjusted to the metabolic demand). Exercise imposes a potent stress on the ventilatory pump: as speed increases, expired minute ventilation (the product of tidal volume and breathing frequency) increases almost linearly: while it averages 80 L/min at rest, it can reach values in the vicinity of 2000 L/min during heavy exercise.25,26 At the walk and trot, respiratory and step frequencies are generally independent, although studies have reported that they can be coupled at a trot. However, this coupling is neither constant nor compulsory27,28 but when it occurs, it seems that the ‘abdominal piston‘ acts in synergy with the respiratory pump28 probably reducing the oxygen cost of breathing. Once the horse gallops, there is an obligatory linkage between stride and respiratory rates.22,27,29 Step and respiratory frequencies averaging 110 to 130/min and tidal volume between 12 and 15 L are reported in fast galloping horses.25,30 The mechanisms underlying the coupling, as well as its physiological significance, are not yet well understood but it is probably due to a mechanical linkage: the visceral piston, the flexion of the back and the loading of the thorax by the forelimbs probably play an important mechanical role in facilitating breathing.29 During protraction of the forelimbs, the rib cage is pulled forwards and outwards, the visceral content stays backwards because of inertia, favoring inhalation (Fig. 27.4). During weight-bearing, the rib cage absorbs forces and is compressed, and the visceral content moves forwards, favoring exhalation (Fig. 27.4). Experimental evidence shows that in galloping horses it is the back flexion rather than the visceral piston mechanism that assists breathing.22 The mechanism obviously gives a mechanical advantage to respiration. These conclusions have nevertheless been challenged by work showing no evidence that the diaphragmatic electrical activity or mechanical output was ‘spared’ as horses ran at progressively increasing intensity such that total diaphragmatic electrical activity and mechanical output were proportional to exercise hyperpnea and independent of the existence of a coupling between gait and respiration.19 On the other hand, in Standardbred horses trotting at 10 m/sec on a 6% slope, tidal volume of about 18 L and respiratory frequency of approximately 90 breaths/min have been reported.31 This breathing strategy is different from that observed in Thoroughbred horses at similar exercise intensities (Table 27.1). However, despite this lack of locomotion/respiration coupling, Standardbred horses do not reach higher expired minute ventilation than do Thoroughbreds, and they are similarly hypoxemic and hypercapnic.31,32 Table 27.1 Ventilatory and arterial gas tension values in trotting and galloping horses. Despite a different combination of respiratory frequency and tidal volume, both are hypoxemic and hypercapnic at high intensity of exercise. At 10m/sec, Standardbreds were running with a slope of 6% and Thoroughbred with a slope of 4° VT: tidal volume; fb: breathing frequency, Ve: expired minute volume; SF: stroke frequency; PaO2 & PaCO2: arterial partial pressure in O2 and CO2. A recent work examining the expired minute volume during the first minutes after exercise in Thoroughbred horses after maximal exercise showed that there is a noticeable hyperventilation, similar to the end-exercise level of ventilation (with a sharp increase in VT and a lower fb) showing that horses can reach their prodigiously high ventilation even when there is no locomotor–respiration coupling.33 Moreover, coupling is not always the rule, even in healthy horses. For example, horses galloping either on the track or on the treadmill, can sporadically show ‘big respiratory cycles‘ which continue for two or three strides.34,35 An extreme value of 29.7 L tidal volume has been observed in a horse decoupling the 1 : 1 step/respiratory frequency ratio for a 2 : 1 ratio.36 Lastly, breathing pattern during exercise can be altered by respiratory troubles. Qualitative and quantitative assessments of respiratory airflow, pattern of breathing and tidal breathing flow-volume loops at rest and during exercise can, in some cases, help to diagnose clinical and subclinical obstructive respiratory diseases.37 Only a portion of the volume of inspired air reaches the area of the lung where gas exchange takes place: this is the alveolar ventilation. The remaining portion, i.e. two-thirds of the minute ventilation at rest, ventilates regions of lung where no gas exchange occurs. This is the physiological dead space ventilation. It includes the conducting airways (anatomical dead space) and the alveoli which are ventilated but not – or only poorly – perfused (alveolar dead space). The dead space to tidal volume ratio (VD/VT) averages 50–60% in the resting horse,38 a percentage twice as high as that reported in humans and dogs. Exercise-induced changes in alveolar ventilation and VD/VT in horses depend on the type of exercise performed.30,39–41 During mild-to-moderate exercise, the dead space volume does not change significantly,39 but because minute ventilation increases, VD/VT decreases (40%) and alveolar ventilation is improved. If the exercise is prolonged at a constant rate, the dead space ventilation increases due to an increase in respiratory frequency and in VD/VT.39,40 This adaptation probably reflects the thermoregulatory role of the respiratory system.41 Lastly, during intense effort, VD/VT decreases from about 60% to 20%,40 with the physiological dead space reduced from 3.5 L at rest to 2.5 L. The distribution of ventilation is not uniform in the lung, even in healthy horses. Two factors can explain this phenomenon. The main one is that the intrapleural pressure changes are not uniform all over the thoracic cage.42 A second reason is the existence of inequalities in regional small airways resistance and/or alveoli compliance: inhaled air preferentially enters the areas of the lungs with low resistive airways and highly compliant alveoli (Fig. 27.5). The resulting ventilatory asynchronism is moderate in healthy horses at rest, and does not have significant effects on gas exchange at low respiratory frequencies. On the contrary, in horses with significant asynchronism (e.g. subclinical obstruction of small airways), this phenomenon can significantly impair gas exchange (Fig. 27.5) especially during exercise, with high respiratory frequency. The lobules which have a long time for filling do not fill adequately before expiration begins and might even ‘rebreath’ the expired air from other regions. Consequently ventilation/perfusion mismatching and hypoxemia result, possibly leading to exercise intolerance. As already mentioned, blood flow is preferentially distributed to the dorsal regions of the lung11 (Fig. 27.2) and this pattern is exacerbated during exercise.12 The greatest perfusion redistribution occurs during moderate exercise intensity, with little further change when intensity further increases. Moreover, there is a considerable heterogeneity in each isogravitational plane,11 which obviously is in disagreement with the gravitational model for distribution of pulmonary blood flow. More than 70% of variation of pulmonary blood flow at rest and during exercise is determined by a fixed spatial pattern that is most likely related to the structural anatomy of the pulmonary vascular tree. Accordingly, despite a redistribution of pulmonary blood flow, heterogeneity of perfusion remains during effort.12 Furosemide, which is frequently used for its putative preventive effect on pulmonary hemorrhage in susceptible horses, slightly attenuates this blood redistribution, but does not influence flow heterogeneity.43 The remaining 30% variation of blood distribution can be attributable to modulation of vascular diameter or other factors, which remain to be elucidated. It must be pointed out that perfusion heterogeneity is greater in horses suffering from recurrent airway obstruction than in healthy horses.44 Vascular diameter is modulated by smooth muscles that respond to vasoactive compounds. A wide range of mediators can modulate vascular smooth muscle tone, and among these epinephrine, norepinephrine, dopamine, prostaglandin and endothelin,45 which is notably potent, are vasoconstrictors, and isoproterenol, bradykinin, histamine, nitric oxide and acetylcholine produce vasodilatation. Lung volume also plays a role in pulmonary blood flow by passively modulating vascular resistance. A distinction must be made between extra-alveolar vessels and alveolar vessels (thin-walled capillaries which perfuse alveoli). The cross-section of extra-alveolar capillaries is dependent on the pressure changes in the interstitial tissues rather than in the alveoli, while the opposite is true for the intra-alveolar capillaries. Consequently, vascular resistance varies with degree of lung inflation, as shown in Figure 27.6. During intense exercise, pulmonary blood flow increases approximately 10-fold.46 The pulmonary right-to-left shunt fraction of cardiac output is approximately 1% at rest and 0.4% during heavy exercise.47 At the same time, mean pulmonary arterial pressure
Lower airway function
Response to exercise and training
Introduction
General overview: structure and function of the lower respiratory airway
Airway
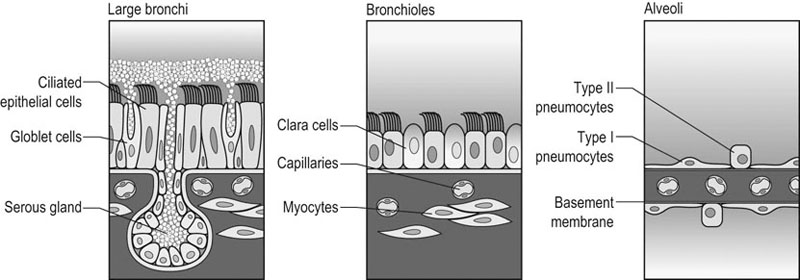
Lung
Blood supply
Thoracic cavity and respiratory muscles
Autonomic innervation
Physiological adjustments to exercise
Ventilation
Ventilatory data and respiratory strategy
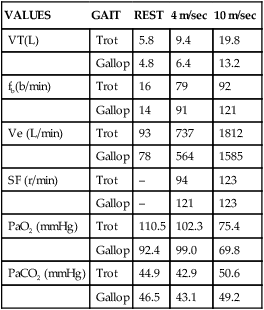
VALUES
GAIT
REST
4 m/sec
10 m/sec
VT(L)
Trot
5.8
9.4
19.8
Gallop
4.8
6.4
13.2
fb(b/min)
Trot
16
79
92
Gallop
14
91
121
Ve (L/min)
Trot
93
737
1812
Gallop
78
564
1585
SF (r/min)
Trot
–
94
123
Gallop
–
121
123
PaO2 (mmHg)
Trot
110.5
102.3
75.4
Gallop
92.4
99.0
69.8
PaCO2 (mmHg)
Trot
44.9
42.9
50.6
Gallop
46.5
43.1
49.2
Partitioning of the respiratory volumes
Ventilation distribution
Perfusion and vascular adjustments
Blood flow distribution
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Lower airway function: Response to exercise and training
