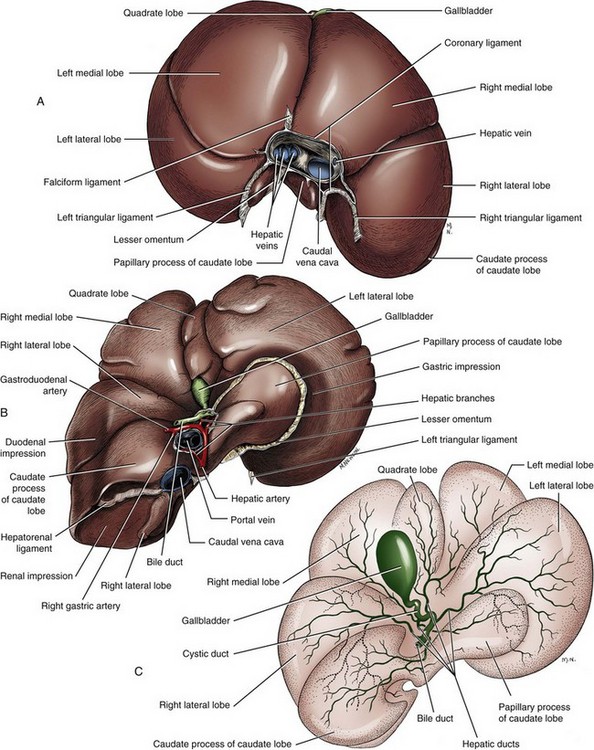
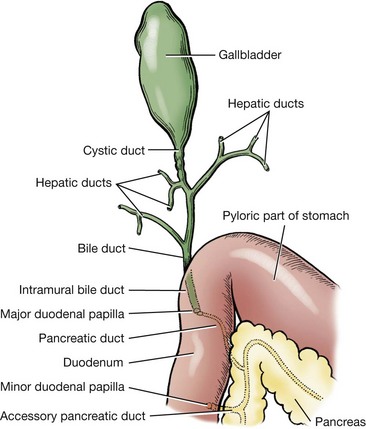
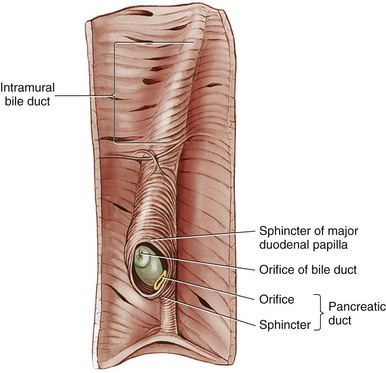
Chapter 95 The liver is housed in the cranial abdomen, well protected from external trauma by the inherent rigidity of the surrounding rib cage. The greater proportion of the liver’s mass lies to the right of the median plane, with a right-to-left proportion of approximately 3 : 2 in dogs.49 The liver is deeply fissured in dogs and cats, an adaptation thought to be related to injury prevention. As the body contorts, fissures allow the lobes to move over each other without risk of parenchymal fractures, which would be a likely sequela to bending forces placed on such a friable organ. The fissures subdivide the liver of dogs and cats into five lobes, four sublobes, and two processes (Figure 95-1).49 The left lobe is the largest and is subdivided into the left lateral and left medial lobes. A substantial cleft separates the two portions of the left lobe, making surgical access to the bases of the left lateral and medial lobes less technically demanding compared with right side approaches.39,55 A deep fissure also separates the left medial lobe from the quadrate lobe and extends from the porta hepatis almost all the way to the esophageal notch.55 The quadrate lobe lies almost on the midline, and its lateral aspect forms one side of the gallbladder fossa. Its attachment to the right medial lobe is substantial, with often only a small fissure separating these two lobes, which makes surgical separation of these two lobes more challenging.39 The right lateral lobe is usually fused at its base with the caudate lobe, which is subdivided into the caudate and papillary processes. The caudate process is the most caudal part of the liver, usually extending to the level of the twelfth intercostal space. The papillary process extends toward the left side, crossing the midline, and is loosely covered by the lesser omentum.55 The liver is loosely tethered to surrounding organs by a series of structures.55 The vena cava runs through the liver and is firmly attached to it. A coronary ligament attaches the liver to the diaphragm and is a continuation of the hepatic peritoneal covering, which is present over the entire surface of the organ except for the region of the porta hepatis. Emanating from the coronary ligament are two right-sided triangular ligaments, a larger one that fuses onto the dorsal part of the right lateral lobe and a smaller one that attaches to the right medial lobe. In most cases, a single left-sided triangular ligament attaches to the left lobe of the liver. The hepatorenal ligament attaches the caudate lobe of the liver to the right kidney. The lesser omentum loosely surrounds the papillary process of the liver and attaches the liver in the region of the porta hepatis to the lesser curvature of the stomach (as the hepatogastric ligament) and the proximal duodenum (as the hepatoduodenal ligament). The epiploic foramen forms a natural opening into the omental bursa and is bordered dorsally by the caudal vena cava and ventrally by the portal vein and lies medial to the caudate liver lobe and to the right of midline. During ventral celiotomy, the epiploic foramen can be accessed by retracting the mesoduodenum to the left side and passing an index finger along the ventral wall of the caudal vena cava in a cranial direction. If the opposing thumb of the same hand is used to compress the tissue between it and the index finger located within the epiploic foramen, simultaneous occlusion of the hepatic artery and portal vein can be achieved, cutting off all inflow of blood to the liver. This is termed the Pringle maneuver. It can be performed for approximately 20 minutes in dogs before hepatic necrosis results.178 Significant metabolic acidosis can be detected in portal blood after shorter periods of occlusion, however.144 Blood supply to the liver comes from the hepatic artery, which is a branch of the celiac artery, and the portal vein. The hepatic artery provides approximately 20% of the blood volume and 50% of the oxygen supply; the portal vein supplies 80% of the blood flow and the remaining half of the oxygen supply.120 At the level of the porta hepatis, the hepatic artery usually branches into two to five branches that penetrate the different lobes of the liver. A right lateral branch usually supplies the caudate and right lateral lobes. A right middle branch usually supplies the right medial lobe, the dorsal part of the quadrate, and part of the left medial lobe. The left branch supplies the left lateral lobe, part of the quadrate lobe, and left medial lobe. The cystic artery to the gallbladder is a branch of the left branch of the hepatic artery. The portal vein is created by the confluence of the cranial and caudal mesenteric veins at the level of the left pancreatic limb.56 Additional tributaries joining the portal vein proximal to its entry into the liver at the porta hepatis include the splenic and gastroduodenal veins, although the latter is absent in cats. Upon entering the liver, the canine portal vein generally divides into a right and left main branch. The right branch supplies the caudate process and the right lateral lobe. The left branch usually gives off a central branch that supplies the right medial lobe and papillary process and then divides into the left lateral, left medial, and quadrate branches, which supply their respective lobes.196 In cats, there are usually three main branches—right, central, and left—that supply the relevant lobes of the right, central, and left divisions of the liver. Six to eight hepatic veins usually drain venous blood from the canine liver into the caudal vena cava. The entry of the most cranial hepatic vein, arising from the left hepatic lobe, is usually located close to the point where the vena cava passes through the diaphragm. Branches from the central division enter the vena cava more caudally, and the branches from the right division are more caudal still and usually located within hepatic parenchyma. The left hepatic vein and branches from the central division are variably covered in hepatic parenchyma. Within the liver, canaliculi drain bile into interlobular ducts. These converge further into lobar ducts that become known as hepatic ducts as they exit the liver parenchyma and form part of the extrahepatic biliary tract (see Figures 95-1 and 95-2). The number of hepatic ducts in dogs varies from two to eight.55 Hepatic ducts converge to form the common bile duct, which enters the duodenum at the major duodenal papilla (see Figures 95-2 and 95-3). The point at which the first hepatic duct joins the cystic duct is the point at which the common bile duct commences. The gallbladder stores and concentrates bile and excretes it into the intestinal tract. Bile first passes through the cystic duct and common bile duct before entering the duodenum through the sphincter of Oddi. The major duodenal papilla is a small, raised mound located on the mesenteric mucosal margin approximately 3 to 6 cm aboral to the pylorus. The location of the major duodenal papilla can be estimated by locating the point at which the distal common bile duct passes through the right limb of the pancreas to join the mesenteric aspect of the descending duodenum. Because of significant intramural passage of the duct within the wall of the duodenum, the papilla is usually 1 to 2 cm aboral from where the common bile duct appears to join the duodenum on its serosal aspect. There are several important differences in hepatobiliary and pancreatic ductal anatomy of dogs and cats that may, in part, explain differences in the clinicopathologic presentation of disease in these two species. In dogs, the common bile duct enters the duodenum at the major duodenal papilla adjacent to, but not conjoined with, the pancreatic duct. Smooth muscle fibers surround the two ducts as they emerge into the duodenum, forming the sphincter of Oddi.53 The accessory pancreatic duct enters at the minor duodenal papilla; this smaller, less obvious papilla is located approximately 2 cm aboral to the major duodenal papilla and, in dogs, is the principal conduit for pancreatic secretions. In cats, the common bile duct and the pancreatic duct conjoin just before their entry into the duodenum at the major duodenal papilla.207 This may be a possible explanation for the frequent concurrence of pancreatic and hepatobiliary disease in cats.84 In addition, only about 20% of cats have an accessory pancreatic duct exiting at a minor duodenal papilla.24,41 Thus, any disease process or surgical procedure that affects the major duodenal papilla has the potential to affect the entire exocrine pancreatic secretion in cats. Pancreatic exocrine insufficiency has been reported as a complication of duodenal lesions involving the major duodenal papilla in cats.206 The liver has a central role in carbohydrate metabolism and maintains plasma glucose concentrations through gluconeogenesis and glycogenolysis. Hypoglycemia can therefore result from severe hepatic disease, although it too usually only occurs after loss of approximately 70% to 80% of hepatic functional capacity.219 The liver synthesizes cholesterol from chylomicrons and lipoproteins in the plasma and stores lipids in the form of triglycerides that are produced from fatty acids. Almost all of the coagulation factors are produced in the liver except for factor VIII and von Willebrand factor (vWF), which are principally produced by the vascular endothelium (some debate remains as to where factor VIII originates). Coagulation abnormalities may result from deficiency of vitamin K, a fat-soluble vitamin that, in the absence of dietary fat emulsification by bile salts, fails to be absorbed. The liver is also responsible for carboxylation of the vitamin K–dependent procoagulation factors II, VII, IX, and X into their active forms. Also produced in the liver are most of the clinically important anticoagulants and fibrinolytic agents such as plasminogen, antithrombin III, α2-macroglobulin, and α2-antiplasmin. Animals with significant hepatobiliary disease should therefore have coagulation screens performed before invasive interventions. Because of the very significant functional reserve capacity of the liver and the fact that many coagulation factors need to be severely depleted (<15% of normal concentrations) before they cause clinically detectable coagulopathies, many animals with significant hepatic disease may still retain normal coagulation screening test results. As few as 2% will hemorrhage spontaneously.48 The liver contributes the largest proportion of the body’s reticuloendothelial function, the part of the immune system primarily responsible for phagocytosis of harmful endogenous and foreign substances. Kupffer cells are hepatic macrophages distributed throughout the hepatic sinusoids. Along with other sinusoidal cells, hepatic Kupffer cells are the principal reticuloendothelial system cell of the liver. With hepatic dysfunction or vascular anomalies that lead to portal blood bypassing the liver, hepatic reticuloendothelial system function may be reduced. Obstructive jaundice has also been shown to impair normal function of the reticuloendothelial system.228 Bacterial endotoxin, anesthetic agents and other drugs, and other particulate matter may not be removed from the systemic circulation when hepatic reticuloendothelial system function is compromised. This can lead to systemic endotoxemia, sepsis, or excessive sensitivity to certain drugs. Regenerative Capacity After Hepatic Resection or Injury Recent advances in cross-sectional imaging have greatly increased the ability for presurgical planning; however, in the case of neoplasia, resectability cannot always be confirmed with preoperative imaging. Extensive tumors or hepatic lesions often require concurrent removal of substantial amounts of normal adjacent hepatic tissues. Fortunately, the normal liver has an incredible regenerative capacity. In experimental studies, normal dogs tolerated acute removal of 65% to 70% of total liver volume, but they did not tolerate 84% removal,154,204 and approximately 28% of dogs survived at least 7 days after 80% hepatectomy.136 Mortality was not related to hepatic failure but rather the inability of the remnant liver to accommodate total portal vein blood flow without development of excessive portal hypertension. As evidence of this finding, dogs with concurrent side-to-side portocaval shunts had a significantly improved survival rate (57% at 7 days) compared with dogs in which no portal decompression was performed (28% at 7 days).136 Posthepatectomy portal pressures greater than 16 mm Hg (~21 cm water) resulted in significantly lower survival and liver regeneration rates.136 Liver regeneration begins within hours and peaks within 3 days; near complete compensatory hypertrophy and hyperplasia on average is reached by 6 days after 70% hepatectomy64 but may take up to 6 to 10 weeks.204 Szawlowski et al.204 demonstrated that repeated partial hepatectomies in healthy dogs could ultimately achieve 95% liver resection under certain circumstances, with only the native papillary process remaining. Liver capacity returns because of compensatory hypertrophy and hyperplasia, and the change in liver volume is a result of the change in liver blood flow, particularly portal blood flow. Disruption in portal perfusion to the liver results in increased hepatic arterial perfusion because of an intrinsic regulatory mechanism in the liver called the hepatic arterial buffer response.97,117,235 The hepatic arterial buffer response is believed to occur secondary to a lack of washout of adenosine (a potent vasodilator) via the portal circulation, which triggers a compensatory increase in arterial perfusion. During partial hepatectomy, splanchnic blood flow remains constant through the portal system, with perfusion through the remaining portal branches increased, resulting in marked hypertrophy of the remaining parenchyma.235 Preoperative portal vein embolization involves obstruction of the ipsilateral portal vein perfusing the segment of liver to be resected. This technique results in hypertrophy of the contralateral segment of the liver and is currently being used in humans before massive liver tumor resections that might otherwise precipitate liver failure because of inadequate remaining hepatic volume or resultant portal hypertension.118 In dogs, liver volumes increased 25% to 33% after portal vein embolization.77 Besides hemodynamic alterations, increases in cytokines (interleukin-6, tumor necrosis factor-α); growth factors (hepatocyte growth factor, epidermal growth factor, transforming growth factor-β); vasoregulators (nitric oxide, prostaglandin E2); and hormones (insulin, estrogen) all contribute to hepatic proliferation and regeneration.235 Several factors have been identified that reduce hepatic regeneration. Biliary obstruction reduces portal blood flow rapidly in dogs and impedes hepatic regeneration.85 Diabetes mellitus also impedes liver regeneration, most likely through decreased concentrations of insulin, one of the most potent hepatotrophic factors in portal blood.235 In one study, dogs receiving 70% hepatectomy and simultaneous pancreatectomy had reduced liver regeneration compared with dogs undergoing hepatectomy alone, and the reduced regeneration was proportional to the volume of pancreas removed.235 In another study, dogs with partial hepatectomies were subjected to varying amounts of nonhepatic splanchnic visceral removal. In that study total pancreatectomy and nonpancreatic splanchnic evisceration resulted in similar decreases in hepatic regenerative response.200 Additionally, total nonhepatic evisceration halted hepatic regeneration; this effect was not reversible with insulin infusion, suggesting a multifactorial role of splanchnic contribution.200 Malnutrition, male gender, and older age also negatively impact hepatic regeneration.235 The most frequent cause of injury to the extrahepatic biliary tract is blunt abdominal trauma after a motor vehicle accident; penetrating wounds from gunshot, stab, or bite injuries have also been reported.159,223 Iatrogenic injury sustained during abdominal surgery associated with either overzealous gallbladder expression, postoperative leakage from a cholecystotomy, cholecystectomy, or choledochotomy incision, or inadvertent laceration of the common bile duct can also result in leakage and subsequent bile peritonitis. In humans, routine use of laparoscopic cholecystectomy for gallstone disease has made iatrogenic injury to the extrahepatic biliary tract a frequently encountered cause of bile peritonitis.7 Such injuries may be seen more frequently in dogs and cats in the future with the increasing use of laparoscopic surgery in veterinary medicine. When leakage occurs as a result of blunt trauma, location of the perforation is almost always within the common bile duct or hepatic ducts; rarely, leakage from the gallbladder has been reported.159,160,223 Tears or transections are usually located within the body of the common bile duct or, less commonly, the cystic duct. Avulsion injuries of the common bile duct from the duodenum or avulsions of hepatic ducts from the common bile duct are also common.159 Knowledge of this lesion distribution is important so that close examination of these areas is performed during surgery. Penetrating injury is a less common cause of extrahepatic biliary tract trauma but has been described in dogs and cats.113,223 With a gunshot wound to the abdomen, tissue within the trajectory of the projectile undergoes crush injury; pathology, however, is not limited to this tract. Kinetic energy transferred to the body when the projectile hits its target leads to the formation of shock waves that radiate to other more distant tissues. Stab wounds generally are low-velocity injuries that are more likely to cause direct laceration or perforation of tissues. Trauma to the extrahepatic biliary tract in cats is exceedingly rare and has only been reported in three cases. Two cats sustained injury from gunshots,113 and a third was hit by a car.9 In the two cats sustaining gunshot injury, one had perforation of the gallbladder, and the other had avulsion of the common bile duct from the duodenum. The cat that was hit by a car sustained a common bile duct avulsion injury. The most common causes of extrahepatic biliary obstruction in dogs include pancreatitis, neoplasia, gallbladder mucoceles, cholangitis, and cholelithiasis.6,58,133 In cats, most commonly a complex of inflammatory diseases that includes pancreatitis, cholangiohepatitis, cholecystitis with or without cholelithiasis, and neoplasia is responsible (Figure 95-4).16,27,61,124,162, Other less common diagnoses in cats include parasitic infection80,106 and diaphragmatic hernia.38 Myriad pathophysiologic mechanisms occur when outflow of bile from the liver is impaired, and many experimental common bile duct ligation models have been studied to demonstrate these effects.148,201,238 After acute ligation in dogs, ultrasonographically detectable common bile duct dilatation and increase in serum bilirubin are present within 24 to 48 hours.148 Dilatation of the lobar and interlobular ducts is evident by days 4 to 6. Pathophysiologic consequences of extrahepatic biliary obstruction that are particularly relevant to surgical patients include hypotension,4 decreased myocardial contractility,68 acute renal failure,45,173 coagulopathies (including disseminated intravascular coagulation),222 gastrointestinal hemorrhage,46 and delayed wound healing.17 The physiologic basis for these complications is poorly understood, but it is hypothesized that the absence of bile salts in the intestinal tract leads to bacterial overgrowth and endotoxin absorption.12 Impaired clearance of endotoxin from reduced hepatic reticuloendothelial function228 results in systemic endotoxemia.12 Endotoxin is a potent renal vasoconstrictor capable of causing acute tubular necrosis.221 Gastrointestinal bleeding may occur because of endotoxin-mediated gastric ischemia and increased acid secretion.46 Decreased fibroplasia and angiogenesis, causing delayed healing in abdominal wounds of jaundiced patients, have also been detected.17 Hypotension and decreased myocardial contractility have been demonstrated, although their cause remains unclear.4,68 Impaired response to vasopressor agents has also been demonstrated in patients with extrahepatic biliary obstruction.62 Although endotoxemia has never been documented in clinical cases of extrahepatic biliary obstruction in small animals, morbidity and mortality in most reports remain high, and anesthetic-related issues, such as hypotension and a lack of response to vasopressor agents, have been reported, especially in cats.27,124 Bile salt supplementation; administration of polymyxin B, cimetidine, and lactulose; and bowel irrigation have all been shown to prevent endotoxemia in human studies.156 The efficacy of such treatment protocols in small animal patients has never been tested. The release of bile salts into the peritoneum is principally responsible for initial pathologic changes that occur with spillage of bile into the peritoneal cavity. Bile salts cause inflammation, hemolysis, and tissue necrosis. Their hyperosmolality leads to significant fluid shifts from the vascular space into the peritoneal cavity, resulting in dehydration and eventually hypovolemic shock. If a source of bacterial contamination is present, the presence of peritoneal inflammation and effusion promotes and sustains growth of bacteria. Normal canine bile is sterile;230 blunt trauma–induced bile leakage is initially more likely to result in sterile peritonitis. Infection, however, can develop as a result of ascending gastrointestinal contamination, intestinal translocation, or colonization by resident hepatic anaerobes. In penetrating injuries, bacterial infection can be introduced via direct inoculation. This is very significant because in the absence of bacterial infection, bile normally causes mild chemical peritonitis. Bacterial infection profoundly worsens the pathology and subsequent prognosis: multiple studies have demonstrated a significantly higher mortality rate in dogs with septic bile peritonitis compared with those with sterile effusion.113,133 The most common underlying causes of bile peritonitis in dogs are trauma, necrotizing cholecystitis, and ruptured gallbladder mucoceles.33,113,172,233 In cats, bile peritonitis is very rare but is usually associated with trauma.9,113 Injuries or lesions of the upper gastrointestinal tract can also result in bile peritonitis because of leakage of bile out of an intestinal perforation close to the major duodenal papilla. Plain radiographs give little specific information in small animals with diseases of the liver and extrahepatic biliary tract. A rough estimate of hepatic size can be made, but plain radiography is relatively insensitive for this purpose.35 Space-occupying lesions in the cranial abdomen may be suggestive of a liver mass or enlarged gallbladder. Approximately 50% of canine choleliths (most frequently composed of calcium bilirubinate) and 80% of feline choleliths (usually composed of calcium carbonate) are radiopaque and may be visible on abdominal radiography (Figure 95-5).52,91,124 Choleliths may be detected in the region of the gallbladder or common bile duct; however, their causative role in extrahepatic biliary obstruction cannot be assessed, and their presence can be entirely incidental. Calcification of the intra- and extrahepatic biliary ducts is a rare, incidental finding on plain radiography.87 The older techniques of cholangiography and cholecystography are rarely used because ultrasonography in most cases provides more information and is technically less demanding and time consuming than cholangiography and cholecystography.29,234 Ultrasonography is an excellent imaging technique to evaluate the liver and biliary tract in small animals.67,149 Traditional ultrasonography discerns focal or multifocal hepatic disease effectively but is less sensitive for the detection of diffuse hepatopathies.67 Diagnosis of the specific hepatic disease process cannot be achieved with traditional ultrasonography alone because the correlation between ultrasonographic appearance and histopathologic diagnosis is poor.60,145,215 For a specific tissue diagnosis, ultrasonography must be used in combination with fine needle aspiration or needle biopsy techniques. These techniques are generally considered safe and are diagnostic in many cases; however, results vary markedly when these samples are compared with larger biopsy specimens from the same patients.36,220 In one study, morphologic diagnosis assigned to needle biopsies concurred with definitive histopathologic diagnosis in only 48% of cases.36 Results of fine needle aspirates agreed with histopathologic diagnosis in only 30% of dogs and 51% of cats in another study.220 Color-flow Doppler can be used to evaluate hepatic vascular anomalies203 or focal reductions in hepatic blood flow, as seen with liver lobe torsion.75 Contrast-enhanced harmonic ultrasonography is a newer modality that detects the harmonic signal produced by intravenous injection of gas microbubbles to evaluate the perfusion patterns of different organs.67 Two studies have described contrast-enhanced harmonic ultrasonography in clinical cases for detection of a variety of hepatic neoplasms.89,151 Ultrasonography is the principal imaging modality for evaluation of the extrahepatic biliary tract.66,103,149 The normal diameter of the common bile duct is approximately 3 to 4 mm in dogs and cats.103,238 One of the earliest signs of extrahepatic biliary obstruction is distension of the common bile duct, which occurs within 48 hours. This is soon followed by distension of the hepatic ducts, and intrahepatic duct distension is present by 1 week in most cases.148 Ultrasonographic distension of the biliary tree does not necessarily indicate active obstruction: long-term persistence of distension can result from a previous episode of extrahepatic biliary obstruction. Monitoring the degree of obstruction over several days may be helpful in discerning acute obstruction from a previous episode. Another option is to inject a synthetic cholecystokinin (sincalide) intravenously. Whereas normal dogs empty 40% of their gallbladder volume within 1 hour of administration, obstructed dogs empty less than 20% of their gallbladder volume within that time.63 Dogs with gallbladder mucoceles frequently present with enlarged gallbladders that have a typical immobile stellate or finely striated ultrasonographic appearance (“kiwi fruit” gallbladder).21 Choleliths can usually be identified by their focal echogenic appearance and acoustic shadowing. The area around the major duodenal papilla is a common location for obstructive lesions and should be evaluated thoroughly for evidence of neoplasia, pancreatitis, or choleliths causing extrahepatic biliary obstruction. Scintigraphy is another modality that can be used to characterize hepatobiliary disease. It is capable of quantifying liver function but has mainly been evaluated in small animals for diagnosis of cholestasis and extrahepatic biliary obstruction.23,71,90,146 Radiopharmaceutical agents used for hepatobiliary scintigraphy in dogs and cats are usually derivatives of 99mtechnetium iminodiacetic acid (mebrofenin or disofenin).23,71,90,146 After intravenous injection in normal animals, these compounds accumulate within the biliary tract and then pass into the intestines through the major duodenal papilla. If the intestines cannot be visualized within 3 hours of the injection of the agent, extrahepatic biliary obstruction is generally considered to be present.23,71 The main disadvantage of scintigraphy is that it is incapable of giving accurate information as to the exact site or cause of obstruction or of differentiating functional obstruction (e.g., from a thickened gallbladder wall or hepatic adhesions) from mechanical. Radiation safety issues, including patient isolation, must also be addressed. Few descriptions exist in the veterinary literature on the use of computed tomography (CT) or magnetic resonance imaging (MRI) evaluation of the hepatobiliary system except for detection of vascular anomalies. Use of CT was compared with ultrasonography for the detection of hepatic metastases from splenic hemangiosarcoma; no obvious superiority of CT could be established for this indication.78 In one study, use of MRI in distinguishing malignant from benign focal hepatic and splenic lesions had a sensitivity and specificity of 100% and 94%, respectively.34 CT and magnetic resonance cholangiopancreatography are new, noninvasive imaging modalities used in humans to provide functional evaluation of the extrahepatic biliary tract and to diagnose leakage of bile from extrahepatic ducts.185 These modalities have not been evaluated in the veterinary literature but may become available in the future. Used extensively for diagnostic and therapeutic interventions in humans, endoscopic retrograde cholangiopancreatography has now been described in dogs and is likely to become more routine in the future.198,199 Biliary and pancreatic ductal systems are imaged by retrograde injection through the duodenal papillae of an iodinated contrast agent. Therapeutic interventions, such as cholelith removal or stent placement, may also be possible after a diagnosis has been established. Minimally invasive stent placement has been described in a small number of healthy dogs and may hold promise in the future for minimally invasive treatment of dogs and cats with certain forms of extrahepatic biliary obstruction.18 The use of this technique in clinical cases of extrahepatic biliary tract disease has not yet been described. The majority of procedures performed on the liver involve risk of hemorrhage that can be life threatening. Preoperative evaluation of coagulation profiles, blood type, and cross-match (if previous transfusions have been given) and availability of blood products or substitutes are critical. The liver is essential for production of procoagulant (coagulation factors, fibrinogen, vitamin K, thrombopoietin) and anticoagulant (protein C, protein S) substances and removal of activated coagulation factors and fibrinogen degradation products; therefore, the presence of hepatic disease can increase risk of hemorrhage or thrombosis. In one report of 42 dogs with confirmed liver disease,177 at least one coagulation abnormality was present in 57% of dogs. In another report of 32 dogs with naturally occurring liver disease,10 abnormal coagulation test results, including prolonged one-step prothrombin time in 53% or activated partial thromboplastin time (APTT) in 41%, were common. In one report,177 coagulation abnormalities appeared to be most commonly associated with chronic hepatitis and cirrhosis. Relatively low concentrations of fibrinogen, protein C, and factors VII and X in dogs with hepatobiliary disease were also reported.177 Cats with liver disease also often have prolonged one-step prothrombin time results and reduced factor VII concentrations.110 Interestingly, in light of the anticipated coagulation abnormalities, an increased bleeding tendency is not clearly seen in human patients, likely because of improved hemostasis from other changes that occur concurrently in patients with liver disease, such as increased concentrations of vWF and decreased concentrations of protein C, protein S, antithrombin, and plasminogen.112 In addition, only 8% to 23% of humans with primary biliary cirrhosis had vitamin K deficiencies, and only 6% of the vitamin K–deficient patients actually had prolonged one-step prothrombin times.112 Although clinical sequelae to prolonged coagulation times are unclear and an increased tendency for hemorrhage in dogs with naturally occurring liver disease has not been demonstrated, pretreatment with fresh whole blood, fresh frozen plasma, or vitamin K in select veterinary patients undergoing liver surgery may be prudent until more evidence-based information becomes available. This may be of particular importance when partial hepatectomies are being performed because liver function may be considerably diminished after surgery. Hypoglycemia is uncommonly associated with end-stage liver disease in veterinary patients but may be a factor in small or otherwise debilitated patients perioperatively. Glucose supplementation should be considered in these patients and for those undergoing extensive hepatectomy. Hypoglycemia was not associated with liver resection of approximately 50% but can occur when 70% of the parenchyma is removed.8 In animals with severe hepatobiliary compromise or those undergoing extensive hepatic resection, preoperative liver function testing should be considered. Plasma ammonia concentrations under anesthesia were unchanged in otherwise healthy dogs after 40% hepatectomy or complete hepatic artery ligation but were significantly increased after 60% hepatectomy.175 Drugs undergoing hepatic metabolization should be avoided when possible. In addition, halothane has been demonstrated to have potential hepatotoxic effects in dogs and results in significantly increased serum liver enzyme activities for at least 2 weeks after anesthesia in healthy dogs compared with dogs undergoing isoflurane or sevoflurane anesthesia.209 Because complete hepatic exposure may require a caudal thoracotomy, the anesthetist should be prepared to ventilate the patient. Animals with large hepatic masses may require ventilation immediately after intubation because of diaphragmatic compression. Intestinal bacteria and endotoxins continually delivered through the portal system are normally removed via the liver’s mononuclear phagocytic system, primarily the Kupffer cells. However, there remains some controversy regarding the presence of a normal bacterial flora in the canine liver.69,147 In one of the more recent studies of normal canine hepatic flora, 60% (12 of 20) of dogs cultured positive with a variety of organisms, including strict anaerobes, strict aerobes, and facultative anaerobes. The most common isolate was Clostridium perfringens followed by Staphylococcus spp. Approximately 50% of dogs had a single isolate, and 50% had multiple isolates.147 In another study of 248 dogs and cats with confirmed hepatobiliary disease,216 cats were identified to have more positive culture results than dogs (14% vs. 5%) and more single isolates (cats; 83% single isolate; dogs, 50% single isolate). This study confirmed the suspicion that the majority of these organisms are of enteric origin (Escherichia coli, Enterococcus spp., Bacteroides spp., and Clostridium spp.) and that the high likelihood of multiple isolates warrants broad-spectrum antibacterial coverage in these patients. Postoperative broad-spectrum coverage should continue until antimicrobial changes can be made based on individual patient culture and sensitivity testing. When obtaining liver cultures, laparoscopic and surgical samples had significantly more positive results (17%) compared with those obtained by percutaneous needle biopsy (4%).216 In addition, when cultures were collected intraoperatively, biliary culture results were significantly more likely to be positive (30%) than hepatic cultures (7%) in the same canine or feline patient.216 In no patient was the hepatic culture result positive with a negative bile culture result. Based on isolates and sensitivity testing, suggested protocols included a combination of (1) fluoroquinolone, penicillin, and metronidazole; (2) fluoroquinolone and amoxicillin–clavulanate; or (3) fluoroquinolone and clindamycin.216 Capsular hemorrhage may be controlled with pressure, but when it is excessive, surgical clips or staples, various hemostatic agents, or vascular occlusion techniques can be used. Common topical hemostatic agents include gelatin sponge (Gelfoam, Pfizer, New York, NY), oxidized regenerated cellulose (Surgicel, Johnson & Johnson, New Brunswick, NJ), cyanoacrylate glue, and fibrin glues.20 Studies in rats examining soft tissue reaction to the presence of these agents determined that gelatin sponges, oxidized regenerated cellulose, and collagen sponges were all well tolerated for up to 45 days and did not interfere with or contribute to wound healing.5 Oxidized regenerated cellulose was completely absorbed by 45 days with no foreign body reaction, and collagen and gelatin sponges were smaller but persistent at 45 days and surrounded by a thin film of fibrous tissue. Interestingly, a number of studies have suggested antibacterial properties of oxidized regenerated cellulose. In one such study, experimentally infected dog splenotomies packed with oxidized regenerated cellulose had significantly reduced bacterial colonization than those packed with gelatin sponge, which had similar bacterial counts as control animals. This characteristic may be because of local pH changes; however, other factors may contribute.44 More extensive hemorrhage may require increasingly invasive procedures. Vascular occlusion techniques can be characterized as (1) control of central venous pressure (CVP), (2) occlusion of liver inflow, and (3) occlusion of liver inflow and outflow. Maintenance of CVP below 5 cm H2O is considered essential for minimizing blood loss during major hepatic surgery in humans and is emphasized by close communication between the surgeon and anesthetist.212 Occlusion of liver inflow implies a temporary or intermittent Pringle maneuver, which provides occlusion of liver vascular inflow and was originally developed to arrest hemorrhage from traumatic liver injuries in humans.176 Follow-up experiments in dogs in which the entire hepatoduodenal ligament, including hepatic artery and portal vein, was ligated resulted in acute death, suggesting this could not be tolerated.176 Demise of the animals is attributed to subsequent portal hypertension and intestinal congestion rather than hepatic ischemia. Interestingly, humans tolerate more prolonged hepatic pedicle clamping because of better intrinsic portosystemic collateral circulation compared with their canine counterparts. This is one of the few circumstances in which dogs have a reduced collateral circulation compared with humans, and care must be taken to avoid applying to our animal patients the safe, tolerated ischemic times reported in humans. Ischemia-reperfusion injury to the liver is enhanced with prolonged inflow occlusion in patients concurrently undergoing hepatic resection or that have biliary obstruction.212 Pringle Maneuver.: An experienced surgeon should be able to identify the epiploic foramen by feel, even if a large mass or hemoabdomen is present, to perform the Pringle maneuver. The epiploic foramen is located at the caudodorsal aspect of the liver near the porta hepatis between the caudal vena cava dorsally and the portal vein ventrally. The caudal aspect of the epiploic foramen is the celiac artery. Placement of a finger through the epiploic foramen and gentle finger pressure simultaneously compresses the portal vein and hepatic arterial supply to the liver, which can temporarily minimize hemorrhage to isolate the bleeding vessels obscuring the surgical site. The Pringle maneuver can be continuous or intermittent; the latter technique, although associated with increased blood loss, likely results in less reperfusion injury. Tolerated inflow occlusion ischemia times in dogs are unclear but are reported to be less than 20 minutes. Care must be taken during interpretation because many of the studies using canine animal models examine inflow occlusion to one segment of the liver, therefore minimizing the resultant portal hypertension that would result if total inflow occlusion was performed.65,114,115,155 Other studies provided simultaneous portal vein decompression that would likely not be performed in the clinical setting. Until further studies are performed, surgeons should consider that brief periods of inflow occlusion in a normothermic setting will likely be tolerated, but animals undergoing inflow occlusion should be monitored closely for signs of portal hypertension. When intractable hemorrhage remains, particularly from a nonresectable tumor, hepatic artery ligation may be performed in certain cases. The blood supply to liver tumors relies primarily on hepatic arterial perfusion (~95%). In comparison, normal liver parenchyma receives approximately 20% of its blood supply from the hepatic artery and can remain viable with portal blood perfusion after hepatic artery ligation.25 Early studies of canine tolerance to hepatic artery ligation resulted in death because of gangrenous necrosis; however, concurrent antibiotic therapy in combination with complete hepatic dearterialization was tolerated in all nine dogs in one study.69 If hepatic artery ligation is performed in an extreme situation, antibiotics should be administered, and ligation should be limited to the lobar arteries when possible; the patency of the portal branches should be confirmed, and prophylactic cholecystectomy should be considered if time and conditions permit. Appropriate treatment plans are based on having an appropriate diagnosis, which is usually determined by evaluation of cytologic or histologic samples of the liver. Numerous sampling methods are available, ranging from fine needle aspiration, to obtain samples for cytologic examination to more invasive techniques for obtaining larger samples for histopathologic examination, culture, and toxicologic evaluation. Although minimally invasive techniques are often preferred, one should be aware of the limitations and risks of each technique in safely providing an accurate diagnosis, particularly when concerning the liver. Patients with liver disease are often debilitated, with prolonged coagulation times and occasionally ascites (because of concurrent portal hypertension), both of which may contribute to bleeding complications. Fine needle aspiration, the least invasive technique, can be performed safely in almost all circumstances; however, even under ultrasonographic guidance, diagnostic accuracy using cytologic evaluation of fine needle aspiration samples has been poor. One study reported overall agreement between fine needle aspiration and histopathologic diagnoses in only 30% of dogs and 51% of cats.220 Cutting needle biopsy instruments (Tru-Cut) provide larger tissue samples that enable histopathologic diagnoses but can be associated with increased risk. Bigge et al.22 reported an overall 22% minor complication rate (relative hematocrit decrease >10% with no required intervention) and a 6% major complication rate (hemodynamic instability requiring transfusion or fluid support, or resulting in death) in 434 dogs and cats undergoing ultrasound-guided cutting needle biopsies of various organs. Thrombocytopenia (<80,000 platelets/µL) was the greatest risk factor for bleeding complications, and dogs with prolonged one-step prothrombin time or cats with prolonged APTT were also more likely to endure complications.22 Vagotonic shock has also been described in cats after liver biopsies with rapid-firing automatic biopsy needles, use of which may be particularly dangerous in patients with extrahepatic biliary obstruction. Some authors have suggested avoiding these techniques in such patients.184 Studies comparing diagnostic samples obtained with cutting needle biopsy techniques compared with wedge biopsy have reported accurate diagnoses in 48% to 83% of cases.36,213 Intraoperatively, a number of techniques are available to obtain adequate tissue samples. When possible, samples from multiple lobes are preferred, even when the parenchyma appears grossly uniform. If patients are undergoing surgery for other reasons or having surgical procedures performed on the hepatobiliary tract, open surgical liver biopsies are obtained. For diffuse liver disease, the easiest procedure to perform is the suture fracture (Figure 95-6) or guillotine technique on the periphery of the liver lobes. These areas are easily accessible, and the vascular and biliary structures are small.183,184 Alternatively, an ultrasonically activated scalpel (Harmonic Scalpel, Ethicon Endosurgery, Cincinnati, OH) or other device can be used to seal the edges of the liver as biopsy specimens are obtained. Vasanjee et al.213 reported resulting hemorrhage after use of ultrasonically activated scalpel was similar to the ligature technique; both techniques resulted in significantly less hemorrhage than using a biopsy punch, biopsy needle, or laparoscopic biopsy forceps. For focal lesions, biopsy punch or wedge biopsies can be performed (Figure 95-7). Hemorrhage was greatest when using a biopsy punch in one study compared with other biopsy techniques.213 Biopsy punch depths should be limited to less than half the thickness of the lobe to avoid damage to larger hepatic vein branches. If bleeding occurs, a gelatin sponge (Gelfoam) can be packed into the hole. The authors often use the same punch to cut similarly sized gelatin sponge pieces to pack into the biopsy site immediately after obtaining the specimen (see Figure 95-7). Wheaton et al.229 described the successful use of canine-derived fibrin sealant to safely control hemorrhage after liver biopsies in dogs. Laparoscopic liver biopsy allows direct visualization of the organ and may enhance targeted diagnosis of grossly abnormal parenchymal lesions.128,137,183 It is a safe and effective technique for collection of high-quality specimens in dogs.15,213 When a liver biopsy is performed in isolation for a diffuse hepatopathy, a single instrument port will suffice in most cases. This is placed under direct visualization in a paramedian position in either the right or left cranial quadrant of the abdomen. A 6-mm trocar-cannula assembly can be placed to accommodate 5-mm cup biopsy forceps. A second port can be placed on the contralateral side if the surgeon plans to use a hemostatic device (vessel-sealing device or ligature) to harvest the biopsy. The simplest way to biopsy diffuse hepatopathies is by use of 5-mm laparoscopic cup biopsy forceps to harvest pieces of liver from the edge of a lobe. The tissue is grasped and gently twisted until it separates from the rest of the lobe. This technique is associated with minimal bleeding in healthy dogs and yields good-quality tissue samples.15,213 It is also possible to coagulate the periphery of the biopsy site with a vessel-sealing device, which has been shown in some studies to reduce hemorrhage.15,213 Several biopsies are usually taken from multiple lobes. If excessive hemorrhage occurs after liver biopsy, a piece of gelatin sponge (Gelfoam) or oxidized regenerated cellulose (Surgicel) can be passed through a port and manipulated into position at the biopsy site to promote clot formation and hemostasis. Before telescope removal, biopsy sites in all cases should be visualized until the surgeon is convinced that all hemorrhage has ceased. Partial hepatic lobectomy can be performed for smaller, more peripheral lesions or when the liver hilum is not easily accessible. After transection of the liver capsule, blunt dissection using the flat, blunt end of a Bard scalpel handle or the inner cannula of a Poole suction tip can facilitate identification and isolation of larger blood vessels and bile ducts. Smaller vessels can be sealed using electrocautery or vascular occluding staples (Hemoclips or Surgiclips), but larger vessels need to be ligated or sealed. Surgical stapling devices have been used for complete lobectomy, but their use for partial lobectomy has not been evaluated.105 Constant et al.37 reported that the use of an electrosurgical vessel-sealing device (LigaSure, Valley Lab-Tyco Healthcare, Boulder, CO) in open and laparoscopic nonanatomic liver resection models in pigs resulted in less hemorrhage and shorter surgical times compared with a finger-fracture method. Application of topical hemostatic agents can be applied to the remaining surfaces if small amounts of venous bleeding persist; however, complete cessation of bleeding should be the goal. The methods described previously can be used if excessive hemorrhage precludes adequate visualization. If hemorrhage is persistent and intractable, a complete lobectomy can be performed. Covey et al.39 recently described a detailed approach to hilar dissection in dogs and the typical arterial, portal venous, hepatic venous, and biliary anatomy for each lobe in seven canine cadavers. Although there were minor individual variations, the arterial and biliary anatomy were mostly consistent, and most lobes had a single lobar portal vein and a single lobar hepatic vein. The authors concluded that the central division of the liver is best removed en bloc because of the anatomy of the hepatic veins. The dissection technique is advantageous in cases in which hepatic tumors encroach upon the liver lobe hilus, preventing stapling device access or placement because of excessive gross disease. Very large tumors may recruit blood supply from other lobes and elsewhere and may similarly prevent hilus access. In a comparison of surgical stapling devices (AutoSuture TA 90, US Surgical Corp, Norwalk, CT) to dissection and ligation techniques for left lateral hepatic lobectomy, Lewis et al.105 found both techniques safe and effective; however, the dissection technique was slower, less complete, and associated with comparatively more microscopic hemorrhage, necrosis, and inflammation. It should be noted that the left lateral liver lobe is the most mobile, and the same technique may not be applicable for other lobes. For stapled lobectomies of the right and central liver divisions, the authors state that extensive dissection and isolation of the hepatic veins is not necessary; however, a right paracostal incision can aid in placement of the stapling devices for certain cases.105 In addition, the gallbladder can be removed with the lobe when necessary. The stapler in Lewis’ study used 3.5-mm staples that close to 1.5-mm inverted B configurations; thus, vessels smaller than 1.5 mm may continue to bleed despite appropriate staple placement. Narrower (30-mm long) vascular cartridges (V3) with smaller staples (2.5 mm closing to 1-mm diameter) in three rows instead of two rows are available and may be preferred but should be limited to smaller patients with more narrow lobar bases.208
Liver and Biliary System
Anatomy
Attachments
Blood Supply
Biliary System
Species Differences
Physiology
Pathophysiology
Traumatic Biliary Tract Rupture
Extrahepatic Biliary Obstruction
Bile Peritonitis
Hepatobiliary Imaging
Abdominal Ultrasonography
Hepatobiliary Scintigraphy
Computed Tomography and Magnetic Resonance Imaging
Endoscopic Retrograde Cholangiopancreatography
Preoperative Considerations for Hepatic Surgery
Hypoglycemia
Anesthesia
Bacteria
Hemorrhage Control during Hepatic Surgery
Capsular Hemorrhage
Extensive Hemorrhage
Inflow Occlusion
Hepatic Artery Ligation
Hepatic Surgical Procedures
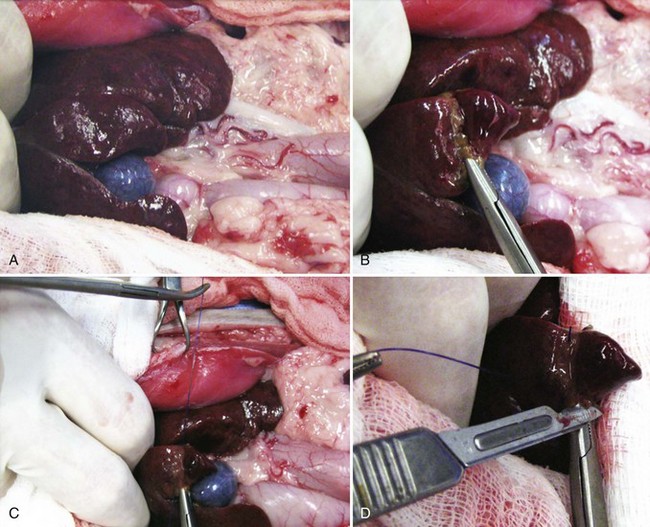
Open Surgical Technique
Laparoscopic Liver Biopsy
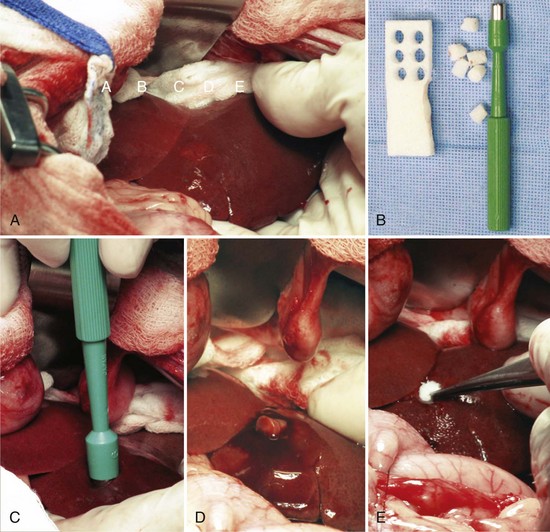
Partial and Complete Hepatic Lobectomy
Partial Hepatic Lobectomy
Complete Hepatic Lobectomy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Liver and Biliary System
Only gold members can continue reading. Log In or Register to continue