4 Leukocyte Disorders
Basic Leukocyte Concepts
Leukogram
Leukocyte responses in the patient are evaluated by the leukogram. The leukogram is the leukocyte portion of the complete blood count (CBC) and includes the total leukocyte (white blood cell [WBC]) count, differential leukocyte count (Diff), and description of WBC morphology. The relative leukocyte differential count (relative Diff) is the percentages of various leukocyte types (i.e., segmented neutrophils [segs], nonsegmented neutrophils [nonsegs], lymphocytes, monocytes, eosinophils, basophils). The absolute differential leukocyte count (absolute Diff) is the number of each type of leukocyte per volume (microliter or liter) of blood. Examination of leukocyte morphology on the stained blood smear is used to determine a relative Diff and detect various abnormalities. Selected hematologic techniques are described in Chapter 2. This chapter is intended to give an overview of understanding the leukocyte response and to answer common questions in diagnosis. Supplemental information regarding leukogram interpretation may be found in other textbooks.18,20,25,35,46
The leukogram usually does not confirm infection, but some patterns, such as a very severe left shift together with very toxic neutrophils, strongly suggest severe infection. Infection is more consistently confirmed by finding the organism and associated inflammatory reaction with cytology (Chapter 16) and culture of tissues that look abnormal on ultrasound, radiographs, or physical exam. Similarly, the leukogram often does not confirm hematologic neoplasia such as malignant lymphoma, while cytology, histopathology, and histochemical and immunologic evaluation of hematopoietic tissues that appear abnormal on ultrasound, radiographs, or physical exam more consistently give a correct diagnosis (see later discussion of hematopoietic neoplasia under Leukemia).
Absolute Versus Relative Differential Leukocyte Counts
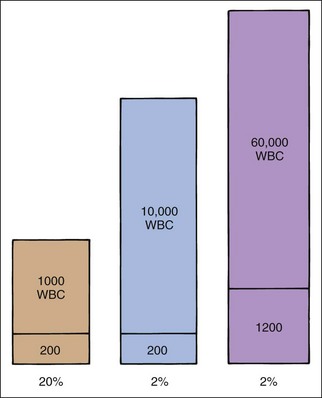
Use of absolute WBC numbers allows more consistent evaluation of leukogram responses than use of relative percentages (Figure 4-1). For example, a WBC count of 10,000 leukocytes/µl with 65% segs has 6500 segs/µl. The 6500 segs/µl is normal, but 65% segs are not always normal. A WBC count of 1000 leukocytes/µl with 65% segs indicates severe neutropenia (i.e., 650 segs/µl). A WBC count of 50,000 leukocytes/µl with 65% segs indicates neutrophilia (i.e., 32,500 segs/µl).
Leukocyte Production, Circulation, and Emigration
Leukocyte Production
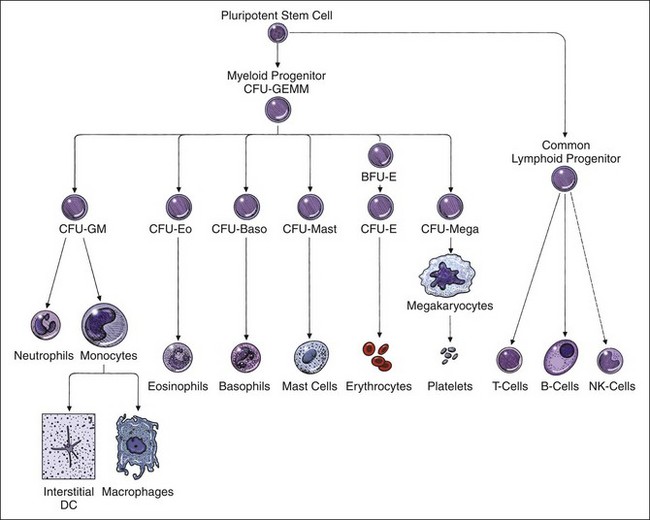
Granulocytes (i.e., neutrophils, eosinophils, basophils) and monocytes are produced in the bone marrow. Although the bone marrow produces some lymphocytes, most lymphocytes are produced by the peripheral lymphoid tissues (i.e., thymus, lymph nodes, spleen, tonsils, bronchial-associated lymphoid tissue, gut-associated lymphoid tissue). Leukocytes develop in the bone marrow from pluripotent and committed stem cells influenced by interleukins and colony-stimulating factors (Figure 4-2).26
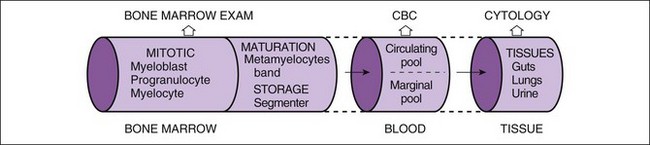
Neutrophils compose the majority of leukocytes in blood, and usually leukocytosis is caused by neutrophilia. Kinetics of neutrophils has been well studied and is best understood. Thus the initial discussion here focuses on neutrophils. Cellular “pools” are used to conceptualize and describe the “location” of neutrophils within the bone marrow and blood and to simplify interpretation of bone marrow and CBC data (Figure 4-3). Bone marrow is divided into two pools. The first, the mitotic pool of myeloblasts, promyelocytes, and myelocytes, provides a steady supply of neutrophils to meet tissue demand for these cells. The second pool is maturation and storage, which consists of metamyelocytes, bands, and segs that lack mitotic ability. Precursor cells undergo progressive maturation and provide a reserve pool of segs to meet sudden increased tissue demands for neutrophils until the mitotic pool increases neutrophil production. It is uncommon in the dog and cat to have leukopenia during inflammatory diseases because this large storage pool is available for rapid release of neutrophils. The maturation and storage pools are combined in Figure 4-3. The most mature stages of neutrophil are preferentially released from the bone marrow into the blood first (segs, bands, metamyelocytes, myelocytes, and finally promyelocytes, in that order). As bone marrow stores of segs are depleted, nonsegs (e.g., bands, metamyelocytes, younger neutrophils) are released into the blood, and a left shift occurs: A left shift is a specific indicator of inflammation, and the severity of the left shift reflects the severity of inflammation.
Leukocyte numbers and morphology within the bone marrow can be evaluated by bone marrow aspiration for cytology and core biopsy for histopathology. Aspirate smears of marrow allow qualitative and quantitative observations regarding cell morphology and maturation. Core biopsy provides the best estimation of bone marrow cellularity and detects stromal reactions (e.g., myelofibrosis, granulomatous osteomyelitis). Chapter 2 discusses the interpretation of a bone marrow examination.
Neutrophil Circulation
When neutrophils are released into the blood, about half hesitatively stick and roll along the endothelial cells (i.e., in the marginal pool) and are not in the central axial flow of blood within vessels from which blood is taken during a venipuncture (i.e., in the circulating pool). Those neutrophils that are in the central axial flow of blood within vessels and are taken into a blood sample (and counted in the WBC count) are said to be in the circulating pool. The marginal neutrophil pool is a “hidden” population associated with the endothelial lining of capillaries, especially the lungs and spleen. The circulating and marginal cell pools make up the total blood neutrophil pool (TBNP). Neutrophils distribute between circulating and marginal cell pools, circulate for a brief period of time (half-life of 7.4 hours), and emigrate from blood vessels into tissues. Shifts between these pools can affect the WBC count, especially in cats. In dogs, circulating and marginal pools are about equal. In cats, the marginal pool is two to three times the size of the circulating pool (Table 4-1). Therefore, if neutrophils are mobilized from the marginal pool to the circulating pool in response to fear, excitement, or strenuous exercise, the neutrophil count can potentially double in dogs and triple in cats. This effect is called physiologic leukocytosis and is seen mainly in young healthy cats.
TABLE 4-1 TOTAL BLOOD NEUTROPHIL POOL, CIRCULATING NEUTROPHIL POOL, AND MARGINAL NEUTROPHIL POOL IN DOGS AND CATS
| DOG | CAT | |
|---|---|---|
| TBNP × 108/kg | 10.2 | 28.9 |
| CNP × 108/kg | 5.4 | 7.8 |
| MNP × 108/kg | 4.8 | 21.0 |
Total blood neutrophil pool (TBNP) in cats is larger than in dogs because of a very large marginal neutrophil pool. The relatively large feline marginal neutrophil pool (MNP), compared with that of the dog, allows a larger potential shift of neutrophils into the circulating neutrophil pool (CNP) with more dramatic leukocytosis during fear, excitement, or strenuous exercise.
Neutrophil Emigration into Tissues
Neutrophils normally spend about 10 hours in the vascular system before emigrating from the blood vessels into the tissues. Emigration is a random (i.e., non–age ordered) and unidirectional (these cells do not return to the circulation) event. In health, neutrophils primarily migrate into the respiratory, digestive, and urinary tracts at a low rate in response to bacteria and other stimuli. Neutrophils lyse quickly in the septic environment of the lumen of the bowel. In inflammation, excessive tissue neutrophils may be visible as exudate or pus (see Chapter 16). In diseases such as enteritis, tissue neutrophils may be hidden from cytologic or gross observation; however, increased tissue demand for neutrophils usually is reflected in the leukogram.
Leukocytosis And Neutrophilia
Differential Diagnosis of Neutrophilic Leukocytosis
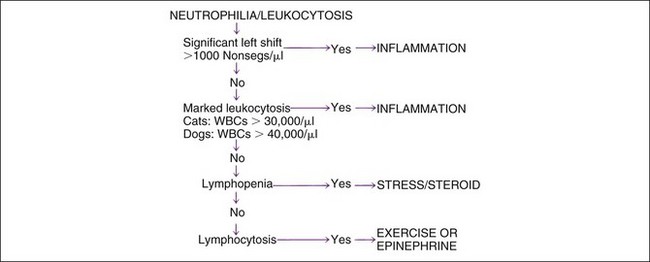
The three most common causes of neutrophilia and leukocytosis are (1) inflammation, (2) stress and corticosteroids, and (3) exercise and epinephrine. Leukemia is relatively uncommon (discussed later) but may cause massive to no increase in the WBC count. Inflammation is most specifically identified by the presence of a left shift, or an absolute increase in nonsegs (Figure 4-4; see later discussion of left shift and hyposegmentation). Inflammation is also suggested by leukocytosis greater than that usually expected with corticosteroid- or epinephrine-associated changes. When mild neutrophilia is present without a left shift, the specific cause of leukocytosis may be unclear. In such instances, the absolute lymphocyte count may be useful. Lymphopenia is commonly associated with an endogenous stress-associated or exogenous glucocorticoid treatment effect. Transient lymphocytosis may be caused by epinephrine-induced splenic contraction or an exercise-associated effect. Persistent lymphocytosis is often caused by chronic immune stimulus. Concurrent processes occur often. For example, inflammation usually causes stress-related lymphopenia. Corticosteroid treatment is very common.
Inflammation
Left Shift
A left shift (increased absolute numbers of immature neutrophils) indicates inflammatory disease in the patient (see Figure 4-4). Identification of immature neutrophils requires blood smear evaluation. Immature neutrophils such bands, metamyelocytes, myelocytes, and younger neutrophils are termed nonsegmented neutrophils (nonsegs) in general. Identification of nonsegs is subjective and varies greatly among microscopists. Most experienced microscopists, who examine blood smears daily, clearly recognize increased immaturity and toxic changes in neutrophils during inflammation. However, the number of bands, metamyelocytes, and myelocytes reported will vary among observers. Exact numbers of these cells are often impossible to determine when severe toxic change is present, in which case a subjective description should be used; for example, “The blood had a severe degenerative left shift with severe toxic change in neutrophils, though exact numbers of each cell type could not be determined.” may be the most honest description. Even in nontoxic reactions the division between segs and nonsegs may be difficult, and the term hyposegmentation is then used to indicate that the neutrophils looked more immature than normal though the number of reported nonsegs was not increased.
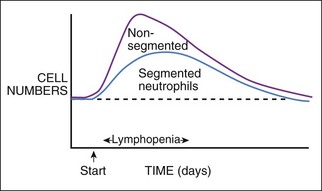
Leukogram Changes in Inflammation
Figure 4-5 shows a likely pattern of leukocyte changes through a typical inflammatory response, with a distinct onset of inflammation and then progressing uniformly to resolution. Changes in individual patients may vary from this typical pattern due to treatment, rupture of an abscess, or the like. The greatest left shift is expected in early stages of the disease process, because as the preexisting bone marrow storage pool is depleted of segs, then more bands and metamyelocytes are released to meet early intense demand. With time, myeloid hyperplasia within the bone marrow expands neutrophil production. When neutrophil production and maturation time are sufficient, mainly mature segs are released into the blood and the severity of the left shift should diminish or disappear. If tissue inflammation stabilizes at a persistent low to moderate level, the bone marrow should reach a production rate sufficient for most neutrophils to mature before release. Thus chronic inflammation may be characterized by little to no left shift and minimal to no leukocytosis. Therefore, chronic or mild inflammation may be difficult to document by leukogram data alone. Acute phase proteins, such as C-reactive protein or serum amyloid A, are sensitive indicators of inflammation and may be used to complement the leukogram. Increased rouleaux (see Chapter 2) in canine blood smears or fever in the patient suggests inflammation. Increased rouleaux formation usually is associated with production of acute phase proteins such as fibrinogen. A normal leukogram appearance does not exclude inflammatory diseases, especially if inflammation is mild or chronic, or only involves a surface (e.g., cystitis).
Bone Marrow Response During Inflammation
Myeloid hyperplasia of the bone marrow is expected with inflammation of greater than 2 to 3 days’ duration. Bone marrow sampling is seldom indicated in patients with inflammatory diseases because a leukocytosis and left shift are usually present, and these indicate the marrow is active. Persistent, moderate to severe leukopenia in a dog or cat with inflammatory disease is unexpected and thus may be an indication to examine the bone marrow for the cause (see Chapter 2).
Prognosis
Leukocyte count criteria, suggesting a poor prognosis, are summarized in Table 4-2 (see also later discussion of Toxic Neutrophils). A degenerative left shift, leukopenia, neutropenia, lymphopenia, leukemoid reaction, or a combination thereof is an atypical, unexpected response to inflammatory disease indicating severe disease, toxemia, severe stress, inadequate bone marrow production, problems interfering with an effective response, or a combination of these. A degenerative left shift has more nonsegs than segs, regardless of total leukocyte count. Finding more immature than mature neutrophils indicates that the bone marrow cannot produce neutrophils at a rate sufficient for them to mature completely prior to release. Either tissue demand for neutrophils has escalated dramatically, cell production is decreased, or both. Severity of change and trend over daily CBCs are important in assessing prognosis. A degenerative left shift, severe neutropenia and leukopenia, lymphopenia, and marked toxic change in most neutrophils often suggest gram-negative sepsis, such as a ruptured intestine or parvovirus enteritis.
TABLE 4-2 LEUKOGRAM FINDINGS INDICATING A POOR PROGNOSIS
| FINDING | REASON FOR POOR PROGNOSIS |
|---|---|
| Degenerative left shift | Tissue demand exceeds bone marrow’s production of neutrophils or causes inadequate time for maturation of neutrophils |
| Leukopenia | Tissue demand exceeds bone marrow’s production of neutrophils |
| Leukemoid reaction | Even excessive numbers of neutrophils cannot correct the problem |
| Toxic neutrophils | Moderate to many, moderately to severely toxic neutrophils are associated with longer hospitalization, higher treatment costs, and increased fatality |
| Severe or persistent lymphopenia | Indicates severe and persistent stress |
A leukemoid reaction is a marked leukocytosis (>50,000 to 100,000 WBCs/µl) due to inflammatory disease and not leukemia. Leukemoid means leukemia-like because of the magnitude of leukocytosis. A leukemoid reaction indicates a poor prognosis because, despite abundant (and actually excessive numbers of) neutrophils, the inflammatory disease is not being corrected. Causes of leukemoid reactions are often severe localized infections (e.g., pyometra, abscess). Additional causes are IMHA, paraneoplastic syndromes with bone marrow stimulation (e.g., metastatic fibrosarcoma, renal carcinoma, rectal adenoma), rare parasitism (e.g., Hepatozoon canis infection), and neutrophil functional defects (canine leukocyte adhesion protein deficiency [CLAD] of Irish setters).39 With pyometra and some walled-off abscesses, there is an anatomic problem preventing healing. Pus and the infectious agent or other cause cannot drain out of the body, and antibiotics may not penetrate the lesion. With CLAD, dysfunctional neutrophils are incapable of correcting common infections even in high numbers. The leukemoid reaction in IMHA seems an exception, but acute massive destruction of erythrocytes and phagocytosis of debris by macrophages is a strong stimulus for an inflammatory reaction.
Toxic Neutrophils
Toxic neutrophils were associated with increased fatality, length of hospitalization, and treatment costs in dogs.3 The prevalence of pyometra, parvovirus infection, acute renal failure, peritonitis, IMHA, disseminated intravascular coagulation (DIC), pancreatitis, septicemia, and neoplastic disorders was significantly higher among dogs with toxic neutrophils. Bacterial infections cause severe toxic changes in neutrophils, such as in secondary bacterial enteritis in parvovirus enteritis. However, toxic neutrophils occur in disease without infection (e.g., IMHA, pancreatitis, chemotherapeutic agents, renal failure). The presence of toxic neutrophils in cats was associated with a significantly higher prevalence of shock, sepsis, panleukopenia, peritonitis, pneumonia, and upper respiratory tract diseases, as were infectious (viral and bacterial) and metabolic disorders.36 Negative findings in cats with toxic neutrophils were milder than with dogs, suggesting that cats form toxic neutrophils with milder diseases than dogs.
Classification of toxemia may be quite detailed3 or more simplified (see Chapter 2). The number of neutrophils that appeared toxic (percentage; or few, moderate, many) and severity of morphologic change should be reported. A few (1+) toxic neutrophils are of minimal importance. However, moderate to many (2+ to 4+) toxic neutrophils should not be ignored (see Figure 2-9). Toxic change in neutrophils, though subjective, is sometimes the only indicator of disease because numerical results of the leukogram appear normal in many cases. A blood smear evaluation by a competent observer should always be included during initial evaluation of a sick patient.
Stress and Corticosteroid Response
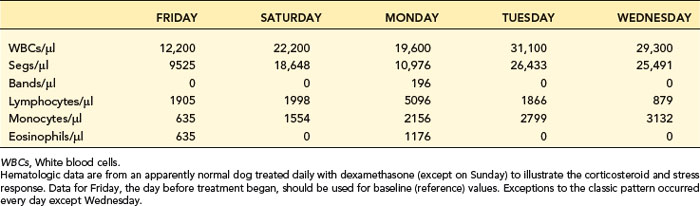
Corticosteroid treatment and stress (endogenous cortisone release) are very common and cause prominent changes in the leukogram (Table 4-3). The classic leukogram pattern from recent corticosteroid treatment or acute stress is moderate leukocytosis with mature neutrophilia, lymphopenia, and eosinopenia. In dogs, mild to moderate monocytosis also may occur (e.g., 2500/µl). Leukocytosis from corticosteroid treatment in dogs may reach a maximum of 30,000 to 40,000 cells/µl, with a predominance of neutrophils, but more commonly there are 15,000 to 25,000 WBCs/µl. Neutrophilia develops in 4 to 12 hours after treatment with a glucocorticoid and returns to baseline values in less than 24 hours. In cats, leukocytosis after corticosteroid treatment is usually a little weaker (e.g., 22,000 WBCs/µl with 18,000 neutrophils/µl), without monocytosis.
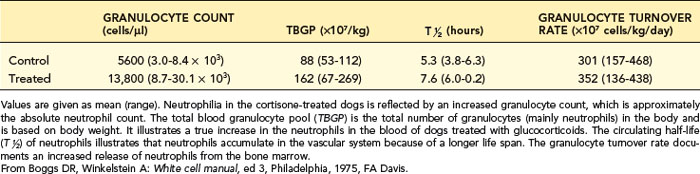
After corticosteroid exposure, the total blood neutrophil pool (TBNP) expands because of decreased emigration of neutrophils from the blood into the tissues and increased release of neutrophils from the bone marrow into the blood (Table 4-4). In addition, neutrophils are shifted from the marginal pool (hidden) to the circulating pool (where they are included in blood collected by venipuncture). A left shift is not expected with stress or corticosteroid treatment because neutrophil release from the bone marrow is usually too mild to stimulate release of bands and metamyelocytes in the presence of a normal bone marrow storage pool of neutrophils.
Acute corticosteroid-induced changes are transient; therefore, one or more of the expected changes may not be seen depending on how long after treatment the sample was taken. Maximal leukocyte changes occur at 4 to 12 hours and may be normalized by 24 hours. For example, Table 4-3 presents hematologic data from a healthy dog treated daily with dexamethasone (except Sunday). One day after initial treatment (Saturday), five of the expected steroid-stress features occurred (i.e., leukocytosis, neutrophilia, no left shift, eosinopenia, monocytosis). Lymphopenia was not present. On day 3 (Monday with no dexamethasone treatment on Sunday) there was lymphocytosis, monocytosis, and eosinophilia most resembling physiologic leukocytosis (described next). Only on day 5 (Wednesday) was the full classic corticosteroid or stress pattern observed.
Exercise and Epinephrine Response
Transient physiologic leukocytosis is noted mainly in young, healthy cats during epinephrine release from fear or after strenuous exercise (e.g., struggling during venipuncture). This response is unlike the steroid-stress response described previously. It is incorrect (at least hematologically incorrect) to call this epinephrine-mediated response “stress.” The TBNP remains unchanged, but a sudden shift of cells occurs from the marginal to the circulating neutrophil pool. This tends to cause a neutrophilic leukocytosis. Contraction of the spleen tends to cause lymphocytosis and polycythemia. There is no increased release of neutrophils from the bone marrow, nor decreased emigration of neutrophils from the capillary beds. Physiologic leukocytosis in cats is greater in magnitude than in dogs because cats have a larger marginal neutrophil pool (three neutrophils in the marginal pool for every neutrophil in the circulating pool; see Table 4-1). Physiologic leukocytosis in cats may be significant; the WBC count often reaches 20,000/µl, and neutrophilia may be overshadowed by lymphocytosis (6000 to 15,000/µl).20 Dogs have such weak physiologic leukocytosis that it is seldom recognized clinically. Physiologic leukocytosis has been noted in research dogs that are bled routinely by the same person and an individual animal’s reference values are available from previous hematology testing. Then, if they are suddenly exercised or frightened (e.g., new blood taker), the mild increases in WBCs, neutrophils, lymphocytes, and packed cell volume (PCV) can be detected.
Leukopenia And Neutropenia
Leukopenia and neutropenia occur infrequently in dogs and cats because they have a large bone marrow storage pool of neutrophils. Leukopenia indicates a poor prognosis. Neutropenia is usually caused by excessive tissue consumption of neutrophils during severe inflammation and/or reduced bone marrow production (Table 4-5). (See also the discussion of bone marrow in Chapter 2 and the discussed of myeloid hypoplasia in the section on Bone Marrow Problems Causing Neutropenia later.) A third cause of neutropenia, which is rare and more theoretical, is a temporary shift of neutrophils from the circulating to the marginal pool, where they cannot be counted. Endotoxin can cause this change. This transient form of neutropenia is actually “pseudoneutropenia,” because the TBNP is unchanged. Rarely, immune-mediated or “steroid-responsive” neutropenia occurs with lupus or some drug treatments.
TABLE 4-5 MAJOR CAUSES OF NEUTROPENIA
| Animals Affected | ||
|---|---|---|
| DOGS | CATS | |
| Consumption of Neutrophils | ||
| Overwhelming sepsis/endotoxemia (important) | ✓ | ✓ |
| Parvovirus enteritis (important) | ✓ | ✓ |
| Salmonellosis | ✓ | ✓ |
| Immune-mediated destruction (rare) | ✓ | |
| Bone Marrow Suppression | ||
| Feline leukemia virus (FeLV) (important) | ✓ | |
| Feline immunodeficiency virus (FIV) | ✓ | |
| Parvovirus (important) | ✓ | ✓ |
| Ehrlichiosis | ✓ | |
| Bone marrow toxicity | ✓ | ✓ |
| Estrogen (endogenous/exogenous) | ✓ | |
| Phenylbutazone* | ✓ | ✓ |
| Phenobarbital | ✓ | |
| Cancer chemotherapy | ✓ | ✓ |
| Irradiation | ✓ | ✓ |
| Leukemia (important) | ✓ | ✓ |
| Myelophthisis/myelonecrosis | ✓ | ✓ |
| Immune-mediated destruction of neutrophil precursors (rare) | ✓ | ✓ |
* Incomplete list of other drugs is in text.
(Courtesy of Dr. M.D. Willard.)
Bone Marrow Problems Causing Neutropenia
Diagnostic testing of patients with persistent, undiagnosed neutropenia includes bone marrow aspiration biopsy, core biopsy, or both. This may detect myeloid hypoplasia, ineffective granulopoiesis, bone marrow necrosis, myelofibrosis, disseminated granulomatous inflammation, leukemia, and other diseases (see Chapter 2). A proper history may reveal treatments or toxins that can affect granulopoiesis (e.g., estrogen or phenylbutazone). Ineffective granulopoiesis confuses many clinicians. The leukopenia is accompanied by normal to increased numbers of myeloid cells (i.e., myeloid hyperplasia). Neutropenia due to myeloid hypoplasia seems more logical. In ineffective granulopoiesis, neutrophils are destroyed in the bone marrow by apoptosis before they mature and are released into the blood. Apoptosis (programmed cell death) occurs in healthy dogs as part of the mechanism to regulate effective neutrophil production. Apoptosis is affected by various mediators, and there is no morphologic change to allow diagnosis of a specific cause. Death of cells within the marrow may be excessive in various infections and drug treatments. Approximately half of feline leukemia virus (FeLV)–positive, neutropenic cats have marked granulocytic hyperplasia indicating excessive apoptosis, whereas half have myeloid hypoplasia suggesting viral destruction of hematopoietic tissue as the cause. Ineffective granulopoiesis may also be an idiosyncratic drug reaction (e.g., phenobarbital).
Damage to or depletion of myeloid cells may cause myeloid hypoplasia (see Chapter 2). Causes of myeloid hypoplasia include parvovirus infection, endogenous and exogenous estrogen toxicosis in dogs, Ehrlichia canis infection, cancer chemotherapy, irradiation, and idiosyncratic reactions to drugs such as phenylbutazone, trimethoprim-sulfadiazine, or chloramphenicol (see Chapter 3). Immune-mediated neutropenia has not been well proven in dogs and cats, but steroid-responsive neutropenias have been described.
Neutropenia may occur from laboratory error. The AutoDiff may be checked by inspection of the instrument’s leukocyte graphics (see Chapter 2) or by screening the blood smear. When in doubt, a full ManDiff should be performed. The total WBC count is seldom wrong, but instrument flags should alert the operator to a problem. Prominent leukocyte aggregation is uncommon but indicates the WBCs were not evenly distributed in the EDTA sample and therefore the WBC count may be incorrect. In such cases new blood samples with different anticoagulants should be obtained and analyzed without delay. Blood specimens from intravenous fluid administration lines may have excessive dilution by the fluid.
Parvovirus Infection
Parvovirus enteritis may be associated with leukopenia, neutropenia with severe toxic changes, and lymphopenia. Parvovirus infects and destroys rapidly dividing cells (e.g., intestinal crypt epithelium, lymphoid tissue, hematopoietic cells). Massive neutrophil exudation through the damaged mucosa contributes to leukopenia, as does hematopoietic cell damage in the bone marrow. Leukopenia is transient and usually early in canine parvovirus disease, so it may be missed without multiple CBCs. Mast cells from the inflamed gut may be detected in blood smears. The mast cells come from damaged intestinal mucosa and do not indicate mast cell neoplasia. Neutrophilic leukocytosis is expected with recovery. During periods of intense granulopoiesis, a leukemoid reaction or other disturbances of normal cell maturation may occur. With complete clinical recovery, baseline leukogram values are regained. Diagnosis of parvovirus enteritis is discussed in Chapter 9. Panleukopenia in cats has a similar hematologic response.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree