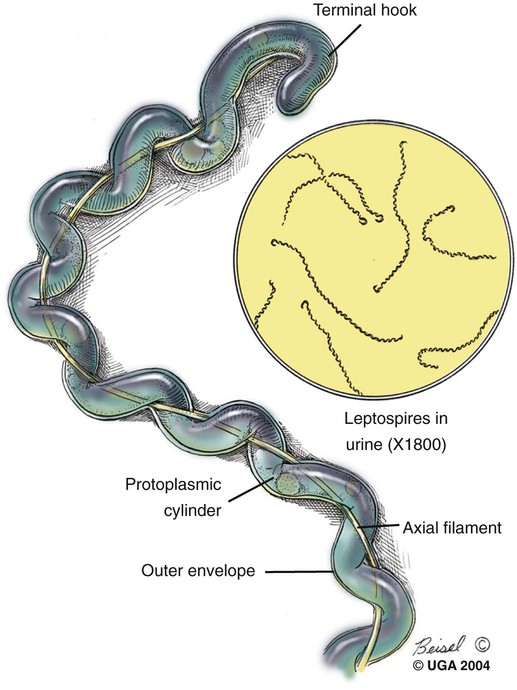
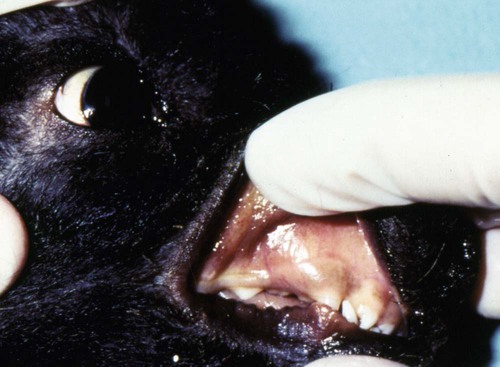
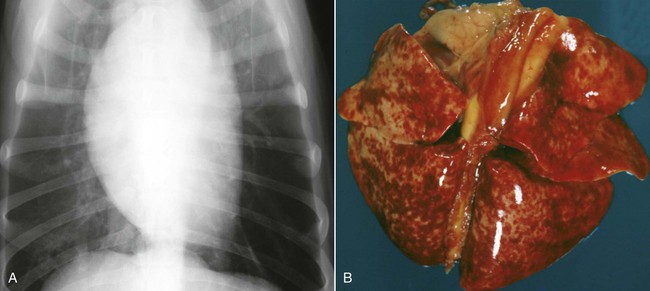
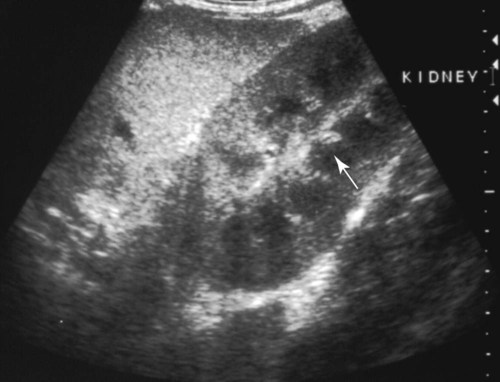
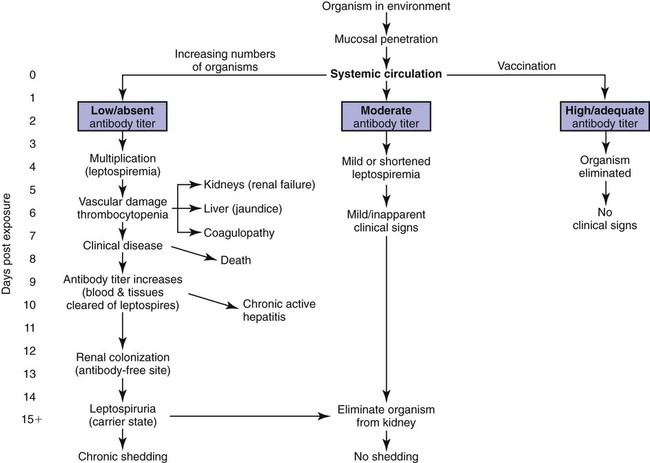
Craig E. Greene, Jane E. Sykes, George E. Moore, Richard E. Goldstein and Ronald D. Schultz Leptospirosis, a zoonotic disease of worldwide significance that affects many animal species, is caused by infection with leptospiral spirochetes of the species Leptospira interrogans sensu lato. Leptospires are thin, flexible, filamentous (0.1 to 0.2 µm wide and 6 to 12 µm long) bacteria made up of fine spirals with hook-shaped ends (Fig. 42-1). They are composed of a cytoplasmic or protoplasmic cylinder surrounded by an inner membrane that is wound around a straight central axial filament composed of two nonoverlapping longitudinally oriented periplasmic flagella that exit the spirochete in subterminal locations. The inner spiral cylinder and overlapping axial filament are surrounded by a layered outer envelope or membrane. Leptospires are motile, making writhing and flexing movements while rotating along their long axis. As with gram-positive bacteria, the peptidoglycan cell wall is closely associated with the inner membrane, as are some lipoproteins (LipL31) and transmembrane proteins such as signal peptidase and ImpL63. The outer membrane is composed of lipopolysaccharide (LPS), multiple antigenic lipoproteins (LipL21, LipL32, LipL36, LipL41), integral transmembrane proteins (e.g., porin OmpL1), and secretins (e.g., GspD). Leptospiral nomenclature and taxonomy is complicated by initial classification by cultural isolation and immunologic reactivity before 1989, with subsequent reclassification using genetic technology. Classically, the genus was divided into two species, L. interrogans sensu lato, containing all pathogenic strains, and Leptospira biflexa sensu lato, with all saprophytic strains from the environment. Within these two groupings, leptospires were identified as different serovars by distinct antibody reactivity to differing carbohydrate moieties in their outer-membrane LPS. Serovars are antigenically, and often epidemiologically, distinct organisms within a given species. More than 250 serovars of L. interrogans have been described and further classified into antigenically related serogroups,59 of which at least 10 are important for dogs or cats. The term serogroup refers to serovars that share common antigens that often lead to cross-reactions with antibody detection methods. Although serogroups currently have no taxonomic basis, they have been historically useful for epidemiologic tracking and understanding of the disease. In cases where diagnosis is made by serologic means and the organism cannot be cultured or genetically identified, serogroup reactivity is technically the highest level of incrimination that can be determined. Furthermore, attempts to correlate specific clinical features of leptospirosis with a given serogroup or serovar can be misleading because serovars from different geographic areas that are immunogenically within one serogroup can have widely differing genetic composition, pathogenicity, and maintenance hosts. Furthermore, serovars within the same serogroup can belong to multiple different leptospiral genomospecies (see next paragraph). For example, serovars within the serogroup Grippotyphosa can be found in both L. interrogans and Leptospira kirschneri.8 Serovars are maintained in nature by numerous subclinically infected wild and domestic mammalian reservoir or maintenance hosts that serve as a potential source of infection and illness for humans and other incidental animal hosts (Table 42-1). Infected incidental hosts develop more severe clinical illness and shed organisms for shorter periods than reservoir hosts. Epidemiologic studies have shown that host preferences can change with time and geographic region of the world. TABLE 42-1 Host Range for Some Isolates of Common Serogroups of Leptospira Infecting Animals With the advent of genetic methods, organisms in the genus Leptospira have been classified into 20 genomospecies on the basis of genetic relatedness (Table 42-2).46,59,172,176 Both pathogenic and nonpathogenic serovars can occur within the same genomospecies in this new genetic classification. The complete genome sequences are available for six leptospiral strains, including two serovars of L. interrogans (Lai and Copenhageni), two strains of L. borgpetersenii serovar Hardjo, and two strains of L. biflexa serovar Patoc. The genome sizes of these organisms range from 3.9 to 4.6 Mbp. TABLE 42-2 Genomospecies of Worldwide Pathogenic Leptospiral Isolates Classified by Serogroupa bOther genospecies not listed here are of intermediate or low pathogenicity or are saprophytes. cThis genospecies was identified in the urine of a large proportion of dogs in Iran where human infections with the same organism were identified.364 In most studies, the common reactivity to serovars in cases of canine leptospirosis reported in the past three decades have been to organisms in serogroups other than Canicola and Icterohaemorrhagiae. Increased serologic testing, since 1980, with additional serovars outside these two serogroups may have contributed to increased recognition of canine leptospirosis. Other serogroups had been incriminated as infecting dogs prior to 1980;* but, they were apparently overlooked with regard to clinical illness, research, and vaccine development. The introduction more than 50 years ago of vaccines containing serovars in the groups Canicola and Icterohaemorrhagiae may also have been responsible for the relative decrease of reports of disease caused by leptospires in serogroups Canicola and Icterohaemorrhagiae. Emergence of disease associated with organisms from other serogroups may stem from additional factors beyond vaccination, including the greater exposure of unnatural hosts such as dogs to wildlife reservoir hosts in rural or suburban environments. Raccoons have been implicated as a source of the serogroup Icterohaemorrhagiae in the northeastern United States283 and Grippotyphosa group infections in the midwestern and northeastern regions of the United States and Eastern Canada,† and they might be involved in serogroup Autumnalis, Bratislava, or Pomona infections in Washington state.71 Cattle or an undetermined wildlife reservoir may be involved in the high prevalence of serogroup Pomona reported in northern California.44,100,100 In contrast to these studies, the predominant serovar in a study from Mexico was in the group Canicola.247 In North Queensland, Australia, prevalence of seroreactivity in clinically affected dogs was greatest against serovars in groups Australis and Zanoni.54 In northeastern Germany, the greatest reactivity in clinically ill dogs was to groups Bratislava, Grippotyphosa, and Pomona.161a In southern Germany, Grippotyphosa and Saxkoebing were the predominant clinically relevant serogroups.98 In Bologna, Italy, reactivity to organisms of serogroup Australis was most prevalent in affected dogs.199 Web Tables 42-1 and 42-2 should be consulted for a review of the serologic reactivity reported worldwide in studies of clinically ill dogs. WEB TABLE 42-1 Clinical Signs of Leptospirosis in Dogsa As, Australis; At, Autumnalis; B, Bratislava; C, Canicola; Co, Copenhageni (previously Icterohaemorrhagiae); G, Grippotyphosa; H, Hardjo; I, Icterohaemorrhagiae; NR, not reported; P, Pomona; Sa, Saxkoebing; Se, Sejroe; Z, Zanoni. aSee Web Table 42-2 for data related to serum biochemical alterations from dogs in these studies. bValues expressed as a percentage of the n value. cFrom IL, USA, 1996–2001: G = 80%, G and B = 20%.43 dFrom Southern Germany, 1990–2003: G = 31%, Sa = 24%, I = 17%, C = 12%, B = 7%, G, B = 5%, I, C =2%.98,130 eFrom GA, USA, 1990–2000: G = 47%, B = 12%, C = 12%, P = 8%, I = 6%, G, P = 6%, H = 2%, B, G = 2%, C, I = 2%, C, G, I = 2%.48,81 fFrom MA, CT, NH, and ME, USA, 1986–1989: G = 41%, P = 29%, P, G = 29%, C = 0%, H = 0% 1 = 0%.280 gFrom Ontario, Canada, 1998–2000: At = 75%; remaining serovars not specified.270 hFrom CA, USA, 1900–1998: P = 44%, B = 25%, P, B = 22%, G, C = 3%, G, P, B, C = 3%, H = 3%.4 iFrom NY, USA, 1980–2001: P = 59%, G = 26%, P, G = 6%, H = 3%, I = 3%, At = 3%.35 jFrom NJ and MI, USA, 1990–1995: P = 20%, P, G, At = 20%, P, G = 13%, At = 7%, H = 7%, G, At = 7%, P, H, At = 7% G, H, I, At = 7% P, G, I, At = 7%, P, G, I, At, B = 7%.123 kFrom Bern, Switzerland, 1991–1996: P, As = 36%, P, As, G = 18%, P, As, I = 18%, P = 9%, P, G = 9%, P, I, G = 9%.320 lGreatest percentage from either physical examination, radiography, or ultrasound. mIncludes coughing, dyspnea; or includes tachypnea. nFrom North Queensland, Australia, 1995–1999: As = 80%, Z =15%, H = 2.5%, Co = 2.5%.219 oFrom Bologna, Italy, 2001–2004: As = 100%.97,199 pFrom upstate NY region, USA, 1996–2002: G = 38%, P =22%, At = 11%, B = 9%, H = 4, C = 1%, G,P = 9%. WEB TABLE 42-2 Selected Clinical Hematological and Biochemical Alterations in Dogs with Leptospirosisa ALP, Alkaline phosphatase; ALT, alanine aminotransferase; NR, not reported. See Web Table 42-1 for data related to clinical signs from dogs in these studies. aValues expressed as a percentage of the n value. In serologic surveys of clinically healthy dogs within geographic regions, the prevalence of seroreactivity among serogroups is much wider than that of clinical disease, suggesting that both subclinical infections and exposures to nonpathogenic serovars occur. Serologic criteria for serosurveys of unvaccinated dogs are lower than those used for diagnosis of the disease; higher criteria are used for vaccine serogroups when a vaccine history exists (see Diagnosis). Many of the sera evaluated by surveys are reactive to multiple serogroups and, as per selection criteria, the dogs have no clinical manifestations of disease. Serologic reactivity in clinically healthy dogs from Bern, Switzerland was to serogroups Grippotyphosa, Pomona, Bratislava, and Australis in addition to Icterohaemorrhagiae and Canicola.320 Results of serologic screening of clinically healthy unvaccinated and vaccinated dogs from the lower Michigan peninsula were reactivity to a variety of serogroups, in decreasing order, including Grippotyphosa, Bratislava, Canicola, Pomona and Icterohaemorrhagiae.322 In Trinidad, sera from clinically healthy dogs from house, farm, or stray backgrounds were most reactive to serogroups Mankarso, Autumnalis, and Icterohaemorrhagiae.3 Sera from clinically healthy shelter dogs throughout Australia were similarly reactive to a variety of serogroups.369 In Poland, the predominant reactive serogroup in healthy dogs was Serjoe.164 In kenneled dogs in Italy, the seroreactivity to serogroups, in decreasing order, was to Bratislava, Grippotyphosa, Canicola, Icterohaemorrhagiae, Sejroe, and Tarassovi with multiple cross-reactions.297 From these studies, the serogroup reactivity and predicted exposure rate are generally lowest to Canicola and Icterohaemorrhagiae, which are used in bivalent commercial vaccines, indicating both potential benefits and concern for immunization practices. In other areas, the prevalence of seroreactivity is low,234 supporting the contention that pathogenic leptospires are not ubiquitous and have wide variations in their geographic prevalence. Despite the presence of leptospiral antibody titers in the feline population,235 reports of overt clinical leptospirosis in cats are infrequent. Although cats seroconvert after exposure to leptospires, they appear to be less susceptible than dogs to both spontaneous and experimental infections.7,80,92,169,351 Serologic studies of feral and shelter cats in the midwestern United States have shown a very low prevalence (less than 3%) of cats with positive results for antibody to serogroups Autumnalis, Bratislava, Canicola, Grippotyphosa, Hardjo, Icterohaemorrhagiae, and Pomona.299a A positive seroprevalence rate of 10% was found in cats in Scotland,7 and 20% for those in Germany.29 Nevertheless, in France, a much higher positive seroprevalence rate of 48% was found in cats with signs of renal or hepatic dysfunction; 87.5% of those with polydipsia and polyuria and 40% of those without polydipsia and polyuria had positive antibody titer results.13 The percentage of positive serogroup reactivity was: Canicola 55%, Sejroe 30%, Australis 12%, and Icterohaemorrhagiae 8%. Leptospires are transmitted between hosts by direct, or more commonly indirect, contact. Direct transmission occurs through contact with infected urine, venereal and placental transfer, bite wounds, or ingestion of infected tissues. Crowding of animals, as may occur in a kennel situation, might theoretically be thought of as an environmental risk of infection; however, such transfer has not been well documented. Recovered or subclinically infected dogs have been shown to excrete viable organisms in urine intermittently for days to months after their initial infection with a variety of serovar isolates included in groups Canicola and Pomona.51,62,141,207 Indirect transmission, which is relatively common, occurs through exposure of susceptible animals to contaminated water sources, soil, and food. Once outside the host, leptospires do not replicate. The spirochete may remain viable for several months in moist soil that has been saturated with urine. Although evidence exists that spirochetes survive in insects and other invertebrate hosts, the significance of this finding with regard to disease transmission is unknown. The indirect transmission of leptospires may increase when environmental factors favoring the survival of leptospires are optimal. Stagnant or slow-moving warm water, although not essential, provides a suitable habitat for spirochetes. Within aquatic environs, leptospires form biofilms on organic and inorganic surfaces that contain colony clusters of organisms.284 Optimal survival in soil is favored by a neutral or slightly alkaline pH. Spirochetes survive only transiently in undiluted acidic urine (pH 5.0 to 5.5), whereas the opposite conditions provide more suitable habitats. Ambient temperatures between 0° C and 25° C favor the survival of leptospires, whereas freezing, dehydration, and exposure to ultraviolet radiation markedly decrease survival. In some geographic regions, there is an apparent increased seasonal incidence of canine leptospirosis in the late summer and fall48,96a,96b,216 or, in the case of regions with rainy cold seasons, the winter.328 Several scenarios exist for the geographic and temporal occurrence of leptospirosis in dogs, and similar observations have been made for the disease in humans. Because of the water requirement, disease incidence or outbreaks often increase during periods of higher rainfall or flooding, respectively, especially in tropical regions. In the United States and Canada, a correlation was found between a greater number of cases of leptospirosis in dogs at veterinary teaching hospitals and higher average rainfall.347 However, in other studies, association with poor drainage or flooding could not be documented.350 Because the exact length of the time between the time of natural infection and development of overt clinical illness has not been determined, it is possible that some cases in the fall and winter months may relate to earlier exposure to concentrated contaminated water sources during dry, warmer periods. In arid areas or during drought conditions, infections of accidental hosts are more common around water sources also visited with increased intensity by reservoir hosts. Infections are observed in rural or suburban dogs that, through activities or utility, are exposed to or allowed to drink standing water, lakes, or streams.100,324,324 In some studies, outdoor, intact male herding dogs, hounds, working dogs, and mixed-breed dogs have been shown at greatest risk in the United States.348,350 The number of reports of disease in urban-dwelling dogs or dogs used to track scents in urban areas has increased, presumably through their contact with infected rodents or their urine.110,331 Polymerase chain reaction (PCR) has detected infection in rodents from such inner-city areas.6,84,84 A potential risk of direct infection to humans exists in these areas (see Public Health Considerations). Highest prevalence of infection in rodents in urban areas has been associated with those residing in sewers.166 Leptospirosis also appears to be of higher prevalence in dogs kept in crowded kennels with unsanitary conditions;297 however, as previously stated, direct transmission of infection between spirocheturic and susceptible dogs and other hosts has not been well documented. One of the authors (CEG) has epidemiologic data results that suggest acquisition of infection in a kennel was related to common source water rather than close housing of infected with uninfected dogs.111 Dogs have been possibly incriminated as a source of waterborne leptospires infecting wild animal populations. One study showed an association between leptospirosis in California sea lions, which were chronically infected with leptospires of serogroup Pomona, and proximity to and density of dog parks that drained to the ocean in their vicinity.243 Further investigations are needed to determine the prevalence of chronic shedding of these spirochetes in dogs, and the interaction between leptospirosis in dogs and wild animal populations. Leptospires penetrate intact mucous membranes of the mouth, nose, or eyes or abraded, scratched, or water-softened skin. Once entering the warmer mammalian body environment, transcriptional changes occur that enhance the spirochete’s pathogenicity.186,202,202 Organisms multiply rapidly after entering the blood vascular space (Fig. 42-2). They then spread and further replicate in many tissues, including the kidney, liver, spleen, central nervous system, eyes, and genital tract. In some experimental studies, the incubation period until development of clinical signs was 7 days, but this may vary according to infecting dose, conditions of natural exposure, infecting strain, and host immunity. With appropriate increases in serum antibodies thereafter, the host clears the spirochetes from most organs, but organisms may persist in the kidney and be shed in urine for days to months (see the earlier discussion of Epidemiology). The extent of damage to internal organs is variable depending on the host immunity, the virulence of the organism, and host ability to contain the infection.114,115,115 Many animals develop more than one of these clinical manifestations, and disease expression can vary among outbreaks and geographic areas. Tissue edema and vasculitis may occur in rapid and severe infections that result in acute endothelial injury and hemorrhagic manifestations. These manifestations are typical of the systemic inflammatory response syndrome (SIRS) associated with sepsis (see Chapter 36). Leptospiral LPS stimulates neutrophil adherence and platelet activation,143 which may contribute to the inflammatory and coagulatory abnormalities that occur. Tissue factor-mediated coagulation activation and impaired fibrinolysis or platelet activation may be results of inflammatory cytokine responses. The exact mechanisms involved are controversial and remain to be substantiated.344,361 LipL32 is the major outer surface lipoprotein of pathogenic leptospires. Much research has been conducted to show its relationship with clinical illness. However, pathogenic lesions with LipL32 mutant were indistinguishable from those produced with wild-type infections.233 Classically, infections of dogs with various serogroups Canicola, Bratislava, and Grippotyphosa have been associated with predominantly renal or hepatic involvement, whereas serogroups Icterohaemorrhagiae and Pomona produce more hepatic disease. In other studies, high titers to serogroup Pomona were associated with severe renal disease.106 Studies attempting to link specific disease manifestations with infecting serogroup have generally been based on serologic diagnosis, which is not consistently reliable for accurate identification of the infecting isolate (see Etiology). Further genetic analysis of isolates from natural disease outbreaks will be needed to determine if clinical signs vary as a result of differences in genomic composition and resultant pathogenicity of serologically related strains. Should recovery occur, it is associated with increased specific antibody in the circulation as early as 7 to 8 days after experimental infection. With complete recovery and elimination of infection, the organisms will eventually be eliminated from the body. However, pathologic changes will persist in the severely affected kidney tissue despite clinical improvement. Recovered dogs that are not given antimicrobial therapy may be more likely to be persistent renal carriers and shed Leptospira in their urine; the duration of shedding has not been determined in all instances and likely varies by the infecting strain (see Epidemiology). Renal colonization occurs in most infected animals because the organism replicates and persists in renal tubular epithelial cells, even in the presence of serum neutralizing antibodies (see Fig. 42-2).61,225 Differential expression of surface antigens of the spirochete may be responsible for its ability to evade host immune responses during the chronic shedding period.224 The organism penetrates the renal capillaries and enters the interstitium, and by 2 weeks after infection, leptospires may be seen within the proximal tubular cells and tubular lumen, which coincides with the onset of shedding. Anti-leptospiral IgG in the renal tubules may also be unable to kill spirochetes because of the absence of complement in the hypertonic interstitium.225 The invasive capacity of leptospires in tissues may be correlated with their pathogenicity because nonpathogenic leptospires do not penetrate cells as readily as do pathogenic leptospires.26 Acute impairment of renal function may result from decreased glomerular filtration caused by kidney swelling that impairs renal blood perfusion. Damage to the endothelium of small blood vessels may result in ischemic damage to the renal parenchyma. In addition, several leptospiral factors have a toxic effect on cells located within the renal parenchyma. Significant renal damage probably results from the leptospiral LPS and other outer membrane protein components. Leptospiral LPS is a potent activator of macrophages, and stimulates secretion of interleukin-1 and interferon by these cells, in addition to augmenting their killing capacity.144 Incubation of peripheral blood mononuclear cells with leptospiral LPS results in the dose-dependent release of tissue necrosis factor-α and interleukin-10. The expression of these cytokines, as well as transforming growth factor-β and the T-cell recruitment cytokine interferon inducible protein-10, has also been detected in hamster models of infection. Tissue necrosis factor-α and inducible protein-10 are proinflammatory cytokines and may play a role in the inflammatory response to leptospires.190 Leptospiral outer membrane proteins, such as LipL32, induce an inflammatory response in renal proximal tubule cells via Toll-like receptors or other ligands.225,343,343 Unsaturated fatty acids in a glycolipid fraction from leptospires specifically inhibit sodium-potassium adenosine triphosphatase (Na+,K+-ATPase), which might account for the urinary potassium wasting observed in many patients with leptospirosis.183,236,236 Endotoxin fractions from L. interrogans have been shown to interfere with renal concentrating mechanisms.52 Several hemolysins have been identified in pathogenic leptospires. The first (sphingomyelinase C) was purified from Leptospira borgpetersenii serovar Hardjo.33 Sphingomyelinase H causes pore formation in target cell membranes and is highly conserved only in pathogenic leptospires.170 Hemoglobinemia, hemoglobinuria, and icterus that develop in cattle with leptospirosis result from a serovar-specific hemolytic toxin produced by isolates from serogroup Pomona. However, hemolysis in other hosts is uncommon despite the presence of other known hemolytic surface moieties. In contrast, decreased osmotic fragility has been detected in canine leptospirosis,292 making hemolysis less likely. A number of fibronectin binding proteins have been identified on the surface of pathogenic leptospires including LipL32, as have surface exposed Lig proteins (LigA and LigB).203 Virulent strains are able to bind complement-4 binding protein, thus interfering with serum neutralization of the spirochete.23 Some of these virulence factors may explain how pathogenic Leptospira evade host defense mechanisms better than attenuated vaccine strains or cultivated isolates. The extent to which these factors cause tissue damage in leptospirosis remains to be investigated. The liver is another major parenchymatous organ damaged during leptospiremia. Profound hepatic dysfunction may occur without major histologic changes because of subcellular damage produced by leptospiral toxins. Because there is no hemolysis, the degree of icterus in both canine and human leptospirosis usually corresponds to the severity of hepatic necrosis. Chronic active hepatitis has been a documented sequela to serovar Grippotyphosa infection in dogs.36 Dogs in a breeding colony of beagles in France had similar disease associated with serogroup Australis infection.2 Presumably, initial hepatocellular injury and persistence of the organism in the liver result in altered hepatic circulation, fibrosis, and immunologic disturbances that perpetuate the chronic inflammatory response. Extensive hepatic fibrosis and failure may result from this process. Leptospirosis should be considered in dogs with hepatic inflammation or fibrosis. Acute lung injury occurs as a result of the effects of the toxins of the organism on pulmonary tissue. Fluid exudation within the lung may result from vasculitis, and rarely, acute severe pulmonary hemorrhage may occur.35,323 The degree of pulmonary impairment is often reflective of the prognosis for recovery.102,196 Geographic variation in the prevalence of acute, severe pulmonary hemorrhage may exist among dogs with leptospirosis. Outbreaks of pulmonary hemorrhage associated with leptospirosis have also been reported in humans and also appear to occur in dogs. Other body systems are damaged during or subsequent to infection that have not been frequently documented in dogs. A benign meningitis, documented in humans, is produced if some leptospires invade the nervous system or cerebrospinal fluid (CSF) during the acute phase of illness. After onset of the antibody response and clearance of tissue organisms, persisting organisms in the central nervous systems of some patients, especially children, may result in development of immune-complex–mediated meningitis.76 Uveitis has also been documented in humans, dogs, and horses, likely through a similar pathogenic mechanism. Box 42-1 summarizes all the clinical features of leptospirosis. Clinical signs in canine leptospirosis depend on age and immunity of the host, environmental factors affecting the organisms, and virulence of the infecting serovar. A comparison of the published historical and physical findings is in Web Table 42-1. Young animals are usually more severely affected than are adults. Large breed (more than 15 kg), outdoor, adult dogs are the most commonly affected.216 In a survey of reported cases from veterinary teaching hospitals in the United States, herding dogs, hounds, and working dogs had a higher risk of infection compared with other breeds.348 However, affected breeds in published reports may vary depending on geographic area, practice setting, or referral base. Small breeds were more frequently affected in cases observed at the teaching hospital at the University of Minnesota (40%) as compared to those at the University of California (16%).328 Peracute leptospiral infections can be exhibited by massive leptospiremia and death with few premonitory signs. In acute infections, pyrexia (39.5° C to 40° C [103° F to 104° F]), shivering, and generalized muscle tenderness are the first clinical signs. Subsequently, vomiting, rapid dehydration, and peripheral vascular collapse occur. Tachypnea, rapid and irregular pulse, and poor capillary perfusion have been noted. Coagulation defects and vascular injury are apparent with hematemesis, hematochezia, melena, epistaxis, and widespread petechiae. Terminally ill dogs become depressed and hypothermic, and renal and hepatic failure does not have time to develop. Icterus or more mild increases in serum bilirubin concentrations may occur in dogs affected with the acute form of the disease (Fig. 42-3). Intrahepatic cholestasis from hepatic inflammation may be so complete that fecal color changes from brown to gray. Dogs with chronic active hepatitis or chronic hepatic fibrosis as a sequela to leptospirosis may eventually demonstrate overt signs of liver failure, including chronic inappetence, weight loss, ascites, icterus, or hepatoencephalopathy. Chronic progressive hepatic disease can occur in the clinical absence of other organ system involvement.2 Pulmonary manifestations include labored respiration and coughing.280 Both interstitial pneumonia and pulmonary hemorrhage with alveolar consolidation have been documented as the cause in humans32,139 and in dogs (Fig. 42-4).30,35,35 Cardiac manifestations may occur in severely affected dogs.199 Rhythm disturbances that can be found on physical examination and electrocardiographic abnormalities have been ventricular tachyarrhythmias. Myocardial damage is suspected based on laboratory findings (see Clinical Laboratory Findings, later) and from similar findings in human leptospirosis.306 Uveitis is commonly recognized in naturally and experimentally occurring leptospirosis in humans and certain animals such as the horse. Panuveitis has been reported in association with leptospirosis in a dog333 and has been found in several dogs examined by the authors of this chapter. Abortion is a clinical problem in mares, cows, and sows; however, there are few reports in dogs. A leptospiral serovar Buenos Aires (serogroup Djasiman) was isolated from an aborted fetus of a seropositive bitch.286 Abortion and infertility was associated with a leptospire serogroup Batavia infection in a dog.85 Based on serologic surveys of clinically healthy dogs, many leptospiral infections in dogs are chronic or subclinical (see the earlier discussion of Etiology).297 The clinical focus for serotesting in dogs has been on those with renal or hepatic dysfunction; however, serologic and microbiologic evaluation for leptospirosis might be performed on dogs with fever of unknown origin, anterior uveitis, or meningitis and on healthy dogs in kennels, multidog households and neighborhoods, or other environs where infection in other members has been documented. Clinical signs are usually mild or inapparent in feline leptospirosis despite the presence of leptospiremia and leptospiruria and histologic evidence of renal and hepatic inflammation.169 More widespread testing of cats with acute hepatic or renal disease for leptospirosis may lead to increased recognition of clinical disease in this species. Hematologic findings in typical cases of canine leptospirosis include leukocytosis and thrombocytopenia. Leukocyte counts fluctuate depending on the stage and severity of infection. Leukopenia, common in the leptospiremic phase, develops into leukocytosis with a left shift. In later stages, white blood cell counts usually range from 16,500 to 45,000 cells/µL. Thrombocytopenia is often a prognostic manifestation of severe cases of leptospirosis, and the degree correlates with the severity of respiratory distress and radiographic pulmonary changes.271 Anemia of mild severity is often observed in dogs with leptospirosis. It is usually nonregenerative, which may reflect: the presence of acute oliguric renal failure with overhydration; decreased erythrocyte production from chronic renal failure or chronic inflammatory disease; or acute (less than 4-day duration) blood loss from tissue thrombosis and/or acute pulmonary or GI hemorrhage. The majority of dogs with leptospirosis are azotemic on initial examination. Increases in serum urea nitrogen and creatinine concentrations are found in dogs with varying severity of kidney disease, although a testing bias may be present because in most retrospective case reports only azotemic dogs were tested (see Web Table 42-2). Hypoalbuminemia can be observed,* (likely from proteinuria or SIRS), and the serum albumin : globulin ratio is usually reduced. The serum globulin concentration may be increased in some dogs.111 The disproportionate hyperglobulinemia may relate to chronic antigenic stimulation from protracted infection, or superimposed dehydration from renal failure, or both. However, concurrent persistent infections associated with hyperglobulinemia should be investigated. Electrolyte alterations usually parallel the degree of renal and GI dysfunction except for marked hypokalemia in many cases. Hyponatremia, hypochloremia, hypokalemia, and hyperphosphatemia are present in most cases, whereas hyperkalemia develops only in dogs with terminal oliguric renal failure. In humans, hypokalemia in the presence of acute renal failure has also been thought to be likely associated with leptospirosis.236 Hypomagnesemia has also been reported in the acute stages of the severe human illness.67 Similar associations have not been documented for the infection in dogs. Mild hypocalcemia is related to hypoalbuminemia and decreased concentration of the protein-bound calcium fraction. Blood pH and serum bicarbonate concentration are reduced in severely affected animals, reflecting metabolic acidosis. Hypoglycemia is occasionally present with severe hepatic failure. Hepatic dysfunction may be apparent in some dogs, but it usually is less dramatic than renal failure or is associated with concurrent renal failure. Liver damage is demonstrated by increased serum alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and alkaline phosphatase activities and bilirubin concentration. The increase in serum alkaline phosphatase activity is often proportionally greater than that of alanine aminotransferase activity. Bilirubin concentration may be increased in serum and urine, and the magnitude is usually proportional to the degree of liver impairment and is a very common finding. Marked bilirubinuria usually precedes hyperbilirubinemia. Serum bilirubin peaks by days 6 to 8 after onset of disease. Increased sulfobromophthalein retention (more than 5%) can be found in acute leptospirosis before the onset of icterus and in dogs that later develop chronic active hepatitis. Increased serum amylase and lipase activities may result from their release from inflamed hepatic and small intestinal tissues and from decreased renal excretion. Increased serum amylase and lipase activities and increased pancreatic lipase immunoreactivity are found in some dogs with apparent secondary pancreatitis. Serum cardiac troponin concentrations have been increased in some dogs, suggesting myocardial damage.199 Serum creatine kinase activity is increased when skeletal muscle inflammation occurs.199 Urinalysis is very often characterized by glucosuria, tubular or glomerular proteinuria, and bilirubinuria and, in some cases, by increased numbers of granular casts, leukocytes, and erythrocytes in the sediment. Some dogs have an increased ratio of protein to creatinine in their urine in the presence of a low-cellularity urinary sediment, which indicates the kidney as the source of the urine protein. The concurrent presence of glucosuria or casts with proteinuria can be explained by acute tubular injury. Findings of predominantly low- and sometimes high-molecular-weight urine proteins are compatible with tubular and glomerular sources, respectively,199,365 although glomerular pathology is not a primary feature of leptospirosis, which is predominantly a tubulointerstitial nephritis. Leptospires will not be observed in the urine without special staining or dark-field microscopy. In experimentally and naturally occurring leptospirosis in dogs, varying degrees of thrombocytopenia, reduced fibrinogen concentration, prolonged activated partial thromboplastin and prothrombin time values, reduced antithrombin activity, increased D-dimer concentration, and increased fibrinogen degradation products have been found, presumably a result of excessive intravascular coagulation.161a,199,238 In many dogs, clotting parameters are within reference limits, or variable thrombocytopenia may be the only abnormality, suggesting compensated hemostatic mechanisms. The degree of thrombocytopenia in dogs with leptospirosis has been correlated with the severity of pulmonary manifestations and higher mortality.161a,271 Although meningitis is not well documented in dogs, increased protein concentration with a predominance of neutrophils would be detected by CSF analysis. In humans, assessment of clinical laboratory findings has allowed prediction of severity and mortality in affected patients.82,196 Dyspnea and severe alveolar infiltrates on thoracic radiography, elevated blood leukocyte counts, thrombocytopenia, oliguria, and repolarization abnormalities on electrocardiograms are all poorer prognostic findings. Similar associations for pulmonary disease and thrombocytopenia271 and for serum albumin, cardiac tropinin I, C-reactive protein/haptoglobin ratio, urinary albumin, and urinary total protein/creatinine ratio199 have been made, suggesting a poorer prognosis for affected dogs. Although coughing and dyspnea are not consistent features of canine leptospirosis, interstitial to nodular alveolar densities, likely associated with pulmonary hemorrhage from endothelial damage and vasculitis, are found in thoracic radiographs of many dogs.30,161a,271 Lesions are most consistently detected in the caudodorsal lung fields. A complete clearance of the lesions occurs in recovered dogs. In decreasing order of prevalence, ultrasonographic abnormalities of the urinary system have included renomegaly, pyelectasia, increased cortical echogenicity, mild perirenal fluid accumulation, and a medullary band of increased echogenicity (Fig. 42-5).93 The medullary echogenic banding is not specific for leptospirosis in dogs and corresponds to a region of renal edema, necrosis, and hemorrhage seen grossly and histologically. Changes suggestive of pancreatic inflammation may also be detected using abdominal ultrasonography.
Leptospirosis
Etiology
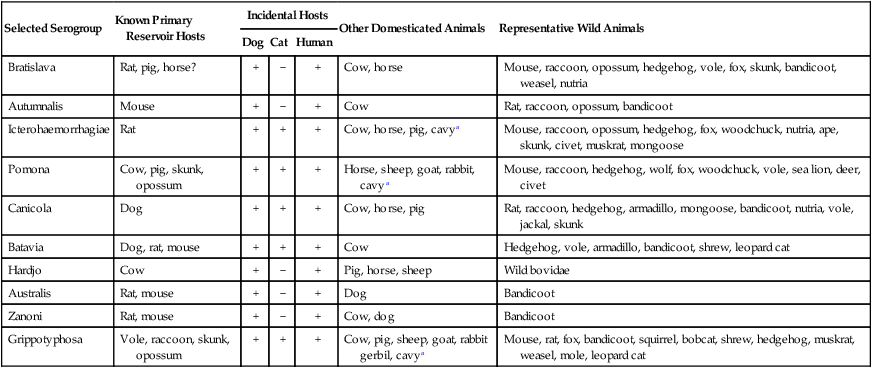
Selected Serogroup
Known Primary Reservoir Hosts
Incidental Hosts
Other Domesticated Animals
Representative Wild Animals
Dog
Cat
Human
Bratislava
Rat, pig, horse?
+
−
+
Cow, horse
Mouse, raccoon, opossum, hedgehog, vole, fox, skunk, bandicoot, weasel, nutria
Autumnalis
Mouse
+
−
+
Cow
Rat, raccoon, opossum, bandicoot
Icterohaemorrhagiae
Rat
+
+
+
Cow, horse, pig, cavya
Mouse, raccoon, opossum, hedgehog, fox, woodchuck, nutria, ape, skunk, civet, muskrat, mongoose
Pomona
Cow, pig, skunk, opossum
+
+
+
Horse, sheep, goat, rabbit, cavya
Mouse, raccoon, hedgehog, wolf, fox, woodchuck, vole, sea lion, deer, civet
Canicola
Dog
+
+
+
Cow, horse, pig
Rat, raccoon, hedgehog, armadillo, mongoose, bandicoot, nutria, vole, jackal, skunk
Batavia
Dog, rat, mouse
+
+
+
Cow
Hedgehog, vole, armadillo, bandicoot, shrew, leopard cat
Hardjo
Cow
+
−
+
Pig, horse, sheep
Wild bovidae
Australis
Rat, mouse
+
−
+
Dog
Bandicoot
Zanoni
Rat, mouse
+
−
+
Cow, dog
Bandicoot
Grippotyphosa
Vole, raccoon, skunk, opossum
+
+
+
Cow, pig, sheep, goat, rabbit gerbil, cavya
Mouse, rat, fox, bandicoot, squirrel, bobcat, shrew, hedgehog, muskrat, weasel, mole, leopard cat
Serogroup
Genomospecies of Isolatesb
Australis
interrogans, kirschneri, noguchii, borgpetersenii
Australis (serovar Bratislava)
interrogans
Autumnalis
interrogans, kirschneri, noguchii, borgpetersenii, santarosai
Canicola
interrogans, kirschneri
Grippotyphosa
interrogans, kirschneri, santarosai
Hardjo
interrogans, borgpetersenii
Icterohaemorrhagiae
interrogans, kirschneri, weilii
Pomona
interrogans, kirschneri, noguchii, santarosai
Saxkoebing
interrogans
Sejroe
interrogans, borgpetersenii, weilii, santarosai
Unclassified
wolffiic
Dogs
Clinical Findingsb
Predominant Serogroup
Grippotyphosa
Autumnalis/Pomona
Pomona
Pomona and Australis
Australis and Zanoni
Australis
Grippotyphosa and Pomona
n =
15c
42d
4.9e
17f
11g
36h
36i
17j
11k
40n
16o
55p
Lethargy/depression
13
81
67
88
90
65
58
88
NR
27.5
88
78
Anorexia
66
76
57
88
81
68
67
65
73
25
69
75
Vomiting
53
57
61
88
81
88
50
71
82
52.5
81
64
Fever
13
36
10
6
13
15
11
6
18
10
19
9
Hypothermia
NR
17
4
18
NR
22
NR
12
36
2.5
38
NR
Dehydration
NR
31
NR
NR
53
NR
36
NR
27
20
NR
26
Weight loss
7
17
37
29
NR
NR
44
NR
9
7.5
NR
35
Polyuria/polydipsia
33
NR
31
35
42
NR
50
18
27
5
NR
31
Abdominal or lumbar pain
NR
19
20
29
65
42
33
35
45
12.5
38
22
Stiffness, arthralgia, myalgia
NR
NR
10
41
35
23
25
24
NR
NR
NR
NR
Renomegalyl
NR
NR
30
31
10
NR
56
20
NR
NR
NR
9
Diarrhea
NR
40
14
24
NR
29
33
6
36
10
38
29
Icterus
NR
45
10
NR
29
NR
33
24
36
65
13
13
Oculonasal discharge
NR
NR
8
18
3
NR
42
NR
9
NR
NR
NR
Posterior paresis, weakness
NR
52
NR
24
NR
NR
39
NR
18
NR
NR
NR
Respiratory signsm
NR
NR
NR
18
35
NR
3
NR
NR
NR
44
NR
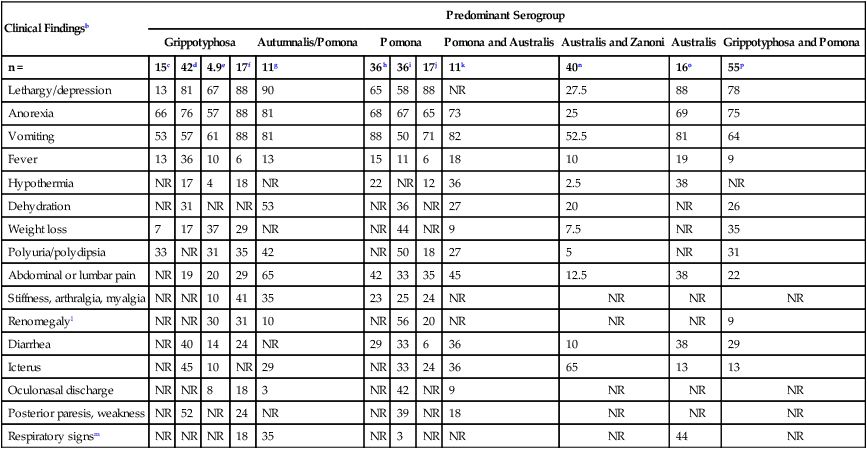
Diagnostic Abnormalities
IL, USA43
Southern Germany98
CA, USA48,81
MA, CT, NH, ME, USA280
Ontario Canada270
CA, USA4
NY, USA35
NJ, MI, USA123
Bern, Switzerland320
Queensland, Australia219
Bologna, Italy199
NY, USA106
n =
15
35
49
17
31
36
36
17
11
18
16
54
Increased serum urea
73
57
91
100
94
100
81
82
55
100
100
93
Increased creatinine
80
NR
91
100
87
100
83
82
55
100
100
93
Hypoalbuminemia
NR
NR
37
18
NR
NR
NR
NR
55
NR
75
35
Hypocalcemia
NR
NR
20
29
NR
NR
NR
NR
NR
NR
13
NR
Hypercalcemia
NR
NR
26
0
NR
NR
NR
NR
NR
NR
19
NR
Hyperphosphatemia
NR
46
74
59
NR
NR
50
47
55
97
94
78
High ALP
33
81
41
59
58
19
56
65
55
94
63
57
High ALT
33
74
27
35
26
NR
33
35
55
61
69
32
Hyperbilirubinemia
33
79
17
24
68 (total)
22
17
41
NR
94
57
41
Hyperkalemia
NR
14
20
18
NR
NR
NR
12
NR
22
31
NR
Anemia
NR
45
29
24
45
NR
33
18
NR
16
38
53
Leukocytosis
NR
81
54
47
58
55b
31
53
73
61
63
37
Glucosuria
NR
NR
10
53
NR
26
9
NR
NR
NR
30
Granular casts
NR
NR
17
18
NR
26
24
0
18
NR
NR
NR
Hematuria (microscopic)
NR
71
13
NR
NR
17
27
42
NR
NR
NR
NR
Proteinuria
NR
71
77
NR
NR
83
28
33
82
NR
94
76
Dilated renal pelvis
NR
NR
27
27
NR
NR
17
13
NR
NR
NR
NR
Hyperechoic renal cortices
NR
24
62
NR
NR
22
33
25
NR
NR
8
NR
Cats
Epidemiology
Dogs
Pathogenesis
Clinical Findings
Dog
Cat
Diagnosis
Clinical Laboratory Findings
Radiographic and Ultrasonographic Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Leptospirosis