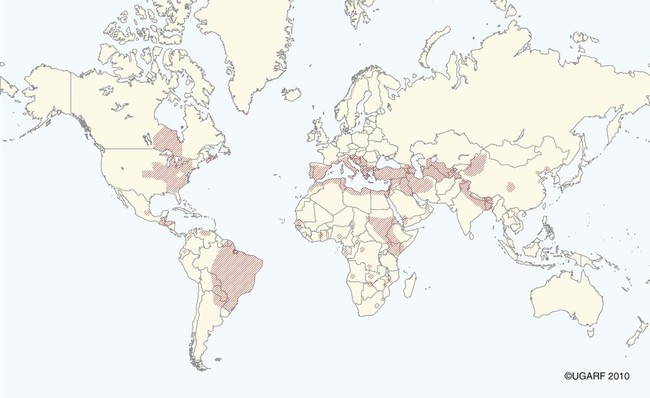
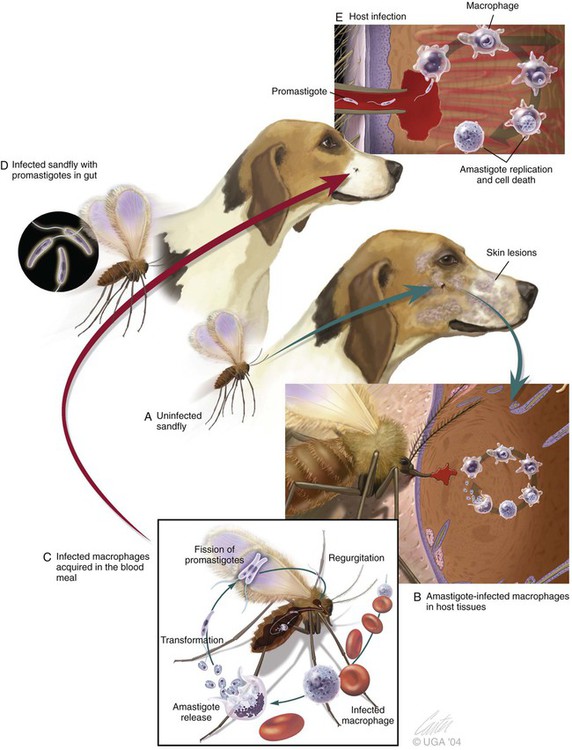
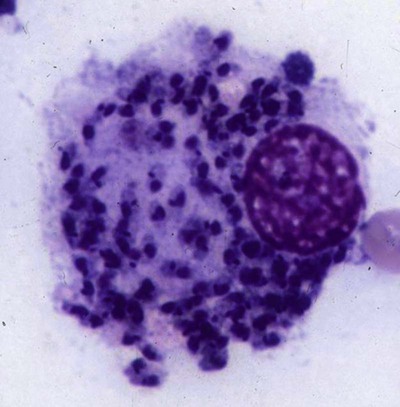
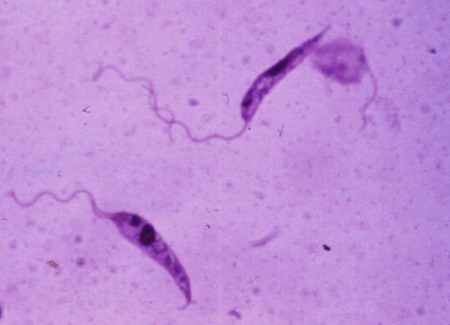
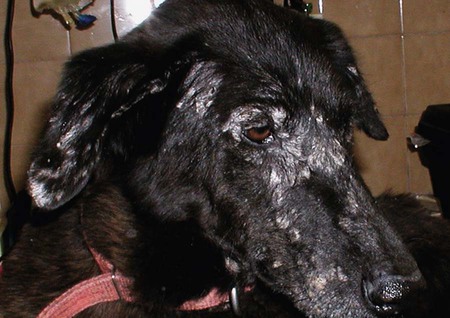
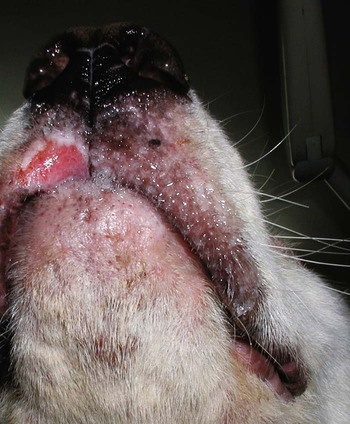
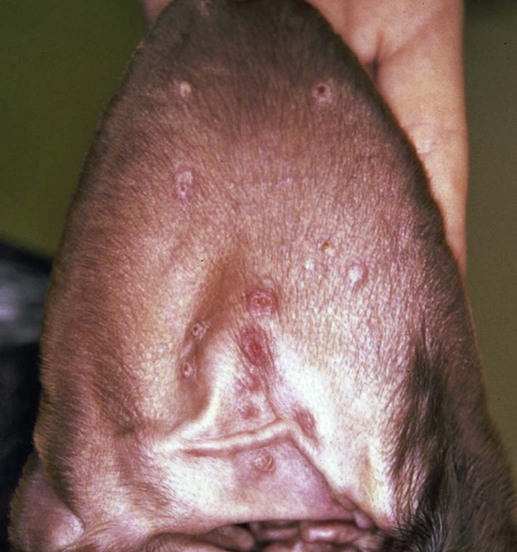
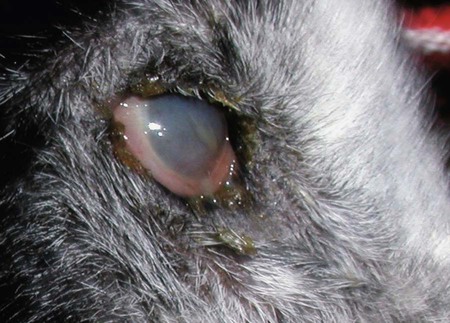
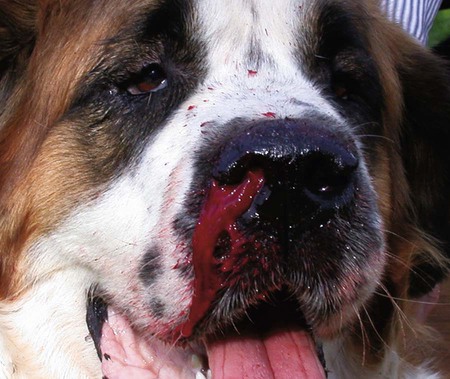
The leishmaniases are a group of infectious diseases that affect humans and domestic and wild animals worldwide and are caused by members of the genus Leishmania. Leishmaniosis is the preferred term for the disease in animals.419 The infection is transmitted by sandflies of the genus Phlebotomus in the Old World and Lutzomyia in the New World. Reservoir hosts vary within different geographic areas and can include domestic or wild animals. Leishmaniosis caused by Leishmania infantum, the most severe disease form, is a frequent cause of clinical illness in dogs in some regions but is less common in cats. Dogs are the major reservoir for canine and human L. infantum (synonymous with Leishmania chagasi245) infection, in an area that stretches from Portugal to China and across South, Central, and parts of North America (Fig. 73-1). Canine leishmaniosis is also sometimes found in nonendemic countries because of international tourists and immigrants who bring infected pets or because of dog importation. As reservoir hosts, dogs can infect feeding sandflies and remain subclinical carriers for extended periods. This chapter presents a global coverage of leishmaniosis, followed by two recognized geographic forms of emerging disease in animals in the New World. This is followed by a section devoted to particular features concerning leishmaniosis in cats. Gad Baneth and Laia Solano-Gallego Leishmaniosis is caused by diphasic protozoans of the genus Leishmania in the class Kinetoplasta and family Trypanosomatidae. The genus Leishmania is divided into the subgenera Leishmania and Viannia based on differences in the location of parasite development within the sandfly. Organisms of the Viannia subgenus such as Leishmania braziliensis replicate in the hindgut, in contrast to the midgut replication characteristic of other Leishmania species (see Canine American Tegumentary Leishmaniosis, later). Several species are recognized within these subgenera, and classification is primarily based on DNA sequence comparisons, electrophoresis migration patterns of isoenzymes (zymodemes), and reactivity to monoclonal antibodies and to membrane-shed antigens. Phlebotomine sandflies of the genus Phlebotomus in the Old World and Lutzomyia in the New World are the natural vectors of leishmaniosis. Sandflies are small insects with a body length seldom exceeding 3 mm.187 The biting activity of sandflies is crepuscular and nocturnal. In the Mediterranean region and Asia, sandflies are primarily active in the warm months, from spring to late fall. In Latin America, some sandfly species are active throughout the year. They do not move long distances, and studies have shown that they are seldom dispersed more than 1 km away from their breeding sites. Many species of sandfly exist, but only some of them act as Leishmania vectors. Different vector species may be found in distinct geographic regions and ecologic niches. Some sandfly species exclusively transmit only one Leishmania species, whereas others are vectors for multiple species. The capacity of different sandfly species to act as vectors appears to be related to the ability of promastigotes to specifically bind to ligands in the sandfly gut. When they do not bind to the sandfly gut, the parasites that initially replicated in the gut lumen are excreted with the sandfly feces and presumably do not reach the critical mass needed to infect a host during a second blood meal.413 As described previously, the natural life cycle of Leishmania infection involves a sandfly vector and a vertebrate host (Fig. 73-2). In vertebrate hosts, Leishmania is found in macrophages in its nonflagellate form, the amastigote. Amastigotes are ovoid or round, 2.5 to 5 µm long and 1.5 to 2 µm wide. In addition to a basophilic-staining nucleus, a rod-shaped, darker-staining kinetoplast is visible with Wright’s or Giemsa stain (Fig. 73-3). Amastigotes multiply by binary fission, then subsequently rupture out of the macrophage to infect new cells. Sandflies can ingest amastigotes when they become engorged with blood from an infected host. In the sandfly gut, amastigotes are released from their host cells, undergo a series of morphologic alterations, transform into the extracellular, flagellated procyclic promastigote form (Fig. 73-4), and replicate. In an appropriate vector, there is sufficient replication and genetically regulated molecular changes in the parasite’s cell surface and detachment from the midgut epithelium. This results in anterior migration of the now-infectious metacyclic promastigotes into the foregut and mouthparts of the vector. Promastigotes are injected with saliva into the skin of a vertebrate host when the female sandfly feeds again. After inoculation into the host, the promastigotes lose their flagella and transform back into amastigotes (see Fig. 73-2). About 30 different leishmanial species are found in various parts of the Old World and New World. Of these, about 20 are responsible for a wide spectrum of clinical illnesses in humans.25 Most Leishmania spp. that infect humans are zoonotic, and only a few are strictly anthroponotic (i.e., transmitted directly from human to human via sandflies). Human leishmaniasis is endemic in 88 countries—66 in the Old World and 22 in the New World—and affects the world’s poorest population living mainly in rural and suburban areas.15 Approximately 12 million humans are infected with leishmaniasis, and some 350 million humans are at risk of acquiring the disease, with a yearly incidence of 1 to 1.5 million new cases of cutaneous disease and 500,000 new cases of the potentially fatal visceral form.106,107 The diseases caused by the various Leishmania spp. in humans are divided into three forms according to their clinical manifestations: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis, and visceral leishmaniasis (VL). Some species cause more than one form of the disease. Anthroponotic visceral leishmaniasis caused by Leishmania donovani is responsible for a large part of the fatalities due to the visceral disease in humans and is present in East Africa, Bangladesh, India, and Nepal.15 Humans are the reservoir hosts for L. donovani, and animals do not appear to play a significant role in the anthroponotic epidemiology of infection,173 although dog ownership has been associated with human leishmaniasis in Ethiopia.40 The main reservoir hosts for Leishmania spp. causing CL and mucocutaneous leishmaniasis in humans are rodents and other wild animal species. Gad Baneth and Laia Solano-Gallego Domestic dogs are considered the main reservoirs for VL in humans in the Mediterranean basin, Middle East, and South America, where L. infantum (L. chagasi) is the causative agent of infection. The low genetic variability of L. infantum, as compared to other species, is consistent with its more contemporary importation to the New World; it is thought to have been introduced by infected dogs from Europe that arrived with early settlers to the Americas.405 Other species of Leishmania have been described to infect dogs in different geographical regions, including L. donovani, Leishmania tropica, L. braziliensis, Leishmania peruviana, Leishmania panamensis,378 and Leishmania amazonensis.400 Based on seroprevalence studies from Spain, France, Italy, and Portugal, it has been estimated that 2.5 million dogs in these countries are infected with L. infantum, and infection is spreading north in Europe, reaching the foothills of the Alps,237 Pyrenees,105 and northwestern Spain.16,265 The number of infected dogs in South America is also estimated to be in the millions, with high infection rates reported from some areas of Brazil and Venezuela.415 In regions with a high infection rate among dogs, the incidence of clinical VL in the general human population has generally been low.418 In contrast, human exposure rates (as determined by the prevalence of specific antibodies or positive leishmanin skin test results), which indicate exposure to Leishmania organisms, may be high.2,5,263,416 This suggests that either subclinical infection or protective immunity exists in humans. Moreover, molecular data obtained in studies carried out on clinically healthy human blood donors from southern France201 and the Balearic Islands333,334 have revealed a high rate of infection (20%). Canine leishmaniosis caused by L. infantum is an important cause of zoonotic disease in many endemic countries and areas, including Spain, Portugal, southern France, Italy, Malta, Greece, Turkey, Israel, Egypt, Tunisia, Algeria, Morocco, Iraq, Iran, the former Asian republics of the Union of the Soviet Socialist Republics, Pakistan, and some parts of China.30,235,247,322,403 Information indicates leishmaniosis may also be transmitted in a part of southern Germany.195 Reports have been made of sporadic cases of canine leishmaniosis caused by importation or transport of infected dogs to countries where sandfly transmission of Leishmania does not occur, such as Holland, the United Kingdom,364 and Sweden.397 Because many dogs in endemic areas harbor subclinical infections, infection rates are often estimated not only by the presence of clinical signs compatible with the disease, but also by serology, demonstration of specific cellular immune responses against Leishmania, and the presence of parasite DNA in tissues as determined by the polymerase chain reaction (PCR). Canine infection rates approach 70% to 90%, as shown by PCR and serology in highly endemic foci, such as the Balearic Islands of Spain,381 the Marseille area in France,45 throughout Greece,204 and the Naples area in Italy.279 This is a much higher prevalence of infection than that associated with disease or determined by serology alone. It is thought that most or all dogs in these areas are infected at some point during their lives.279,381 A low proportion of dogs succumb to the infection and develop disease, whereas the majority of dogs are resistant and harbor the pathogen subclinically.31,378 In other areas, infection is not as highly prevalent, possibly because of less favorable environmental conditions for transmission and vectors.265,423 Age seems to be a risk factor that influences the development of disease. The age distribution of clinical disease has two peaks, one of young dogs (2 to 4 years old) and another in older (more than 7 years old) dogs.257 Data from prevalence studies indicate that several dog breeds, including boxer, cocker spaniel, Rottweiler and German shepherd, are apparently more susceptible to disease.147,257,257 Other breeds that have evolved in endemic areas, such as the Ibizan hound, rarely develop the disease and present a protective predominant cellular immunity.380 In Europe and South America, sensitive xenodiagnostic studies, involving feeding of vector sandflies on dogs, have demonstrated that dogs with or without clinical illness and with serum antibody titers to Leishmania are infectious to a high proportion of feeding sandflies.320 The percentage rate of infected sandflies on a given dog increased with the presence and severity of its clinical signs, high anti-Leishmania antibody level and decrease in CD4+ T-cell count.81,89,166,255 These findings corroborate that clinical disease and high anti-Leishmania antibody levels are positively correlated with high parasite burdens.229,328 Risk of sandfly-borne transmission is reduced as parasite loads decline after treatment.179,332 Although L. infantum is naturally transmitted through the bites of sandflies, other modes of transmission are possible. In utero transmission from a dam to its offspring has been documented in a few clinical reports96 and under experimental339 and natural297 conditions.109 Venereal transmission of leishmaniosis from infected males to healthy bitches has been documented.366 Transmission by hematophagous arthropods other than sandflies has been investigated but not proven to have epidemiologic significance. Rhipicephalus sanguineus ticks have been shown to acquire Leishmania organisms in their gut after feeding on infected dogs83; however, the tick’s competence as a vector has not been confirmed.93b In addition, fleas have been proposed as alternative vector of Leishmania transmission but proof of transmission is still lacking.84,123 Transmission of infection by blood transfusion has been reported150,288 and is of special concern in areas where blood donors are subclinical carriers of infection.99,394 Direct dog-to-dog transmission has been suspected in areas where sandfly vectors are apparently absent.117 Modalities of transmission other than by sandflies should be further investigated; however, it is not known whether they play an important role in the natural history and epidemiology of leishmaniosis.31 Canine infection occurs mostly in periurban or rural areas; however, canine and human infections have been reported in urban areas and pose a threat to the well-being of dogs and humans.88,266 The potential importance of reservoir hosts, other than domestic dogs, in the epidemiology of L. infantum infection is less well known.320 L. infantum infection has been reported in many domestic animals from the Old and New Worlds.320 High prevalence of infection based on positive serum antibody titers and PCR results has been reported in domestic cats in some areas of Europe238 and Brazil.94,95 Clinical disease has been reported in cats.175,305,314,342 Infections have also been reported infrequently in horses in Europe,335,377 and cellular immunity tests in equines have shown that exposure is more frequent than clinical manifestations of infection.122 Serologic responses of pigs to L. infantum have been described in Brazil.262 L. infantum infection has been reported from a wide range of wild carnivores and rodents in the Old World, including a seal.320 In the New World, infection has been detected in crab-eating foxes (Cerdocyon thous) and in a range of other carnivores and rodents, including a bat.320 High prevalence rates of infection have been documented in populations of foxes in Europe and South America.320 Clinical disease has been reported in wild canids.42,213 The majority of reports on other domestic or wild mammals have described subclinical infections. Only a few studies have examined the ability of hosts other than domestic dogs to transmit L. infantum infection to sandflies by xenodiagnosis.320 The ability to transmit Leishmania to feeding sandflies has been confirmed in humans, black rats, domestic cats, crab-eating foxes, and opossums, which thus have the potential to act as primary or secondary reservoirs.320 However, infected crab-eating foxes in Brazil were found to have low rates of transmission to the sandfly Lutzomyia longipalpis. Similar results were found in humans, who were not considered to be important as reservoirs of leishmaniosis in Brazil.82,320 L. tropica occurs in the Old World and is a rare cause of infection in dogs. L. tropica is an important agent of CL in humans in some parts of the Middle East and Africa. It also caused VL in American soldiers returning from the Persian Gulf after the 1991 Operation Desert Storm219 and in patients from India.343 It has been sporadically found in dogs with dermal lesions from Tunisia and Morocco,104 in another dog from Morocco with visceral involvement and no dermal abnormalities,168 and in a dog from Iran.261 As the sandfly bites, Leishmania promastigotes are transferred with the sandfly saliva into the vertebrate host’s skin. The promastigotes are then phagocytized by macrophages and multiply as amastigotes within phagolysosomes that separate them from the host cell defense mechanisms. When the macrophage ruptures, freed amastigotes penetrate additional host cells and disseminate from the local bite site. They travel throughout the host’s body but primarily to the hemolymphatic organs such as lymph nodes, spleen, bone marrow, and liver and remote dermal areas to establish a systemic infection. Interestingly, the distribution of Leishmania amastigotes in fetal organs transmitted in utero is similar to that observed in adult dogs and is mostly also in lymphoreticular organs.297 As mentioned previously, not every dog naturally or experimentally infected with Leishmania develops disease.189,309 The immune responses mounted by dogs at the time of infection and thereafter appear to be the most important factor in determining whether they develop a generalized infection and whether and when the infection will progress from a subclinical state into clinical disease. Infection initially has no apparent signs but later might progress into clinical disease unless the replication of amastigotes is halted by immune mechanisms. Dogs that are able to resist an infection by either resolving it and eliminating the parasite or restricting the infection and remaining subclinical for long periods are considered clinically resistant. Animals that are predisposed to developing disease after infection are considered susceptible. However, subclinical infection is not necessarily permanent, and factors such as immunosuppressive conditions or concomitant disease can break the equilibrium and lead to the progression of clinical disease in dogs.378 This has also been observed in human patients with acquired immunodeficiency syndrome and Leishmania co-infection.12 The innate, or nonspecific, immune response is the first line of defense encountered by Leishmania parasites when entering the susceptible host. One of several suggested mechanisms by which Leishmania parasites evade the innate host immune defenses involves the ability of amastigotes to survive and replicate within macrophage phagolysosomes by producing compounds such as lipophosphoglycans that inhibit phagosome maturation.344 Specific immune responses play a major role in susceptibility to infection. An experimental model of CL in mice infected with Leishmania major has shown that a susceptible mouse strain, which typically develops a T-helper (Th) type 2 (Th2) cellular response, succumbed to infection.169,359 This Th2 response resulted in secretion of specific cytokines such as interleukin (IL)-4 and IL-10 and production of a significant antibody response. Other mouse strains that respond with a different set of cytokines, including interferon (IFN)-γ and IL-12, typical of a Th type 1 (Th1) response are resistant to infection.169,359 IFN-γ secreted from T cells activates macrophages for the elimination of parasites. This general concept derived from the experimental CL model of a Th-cell dichotomy—in which one type of Th-cell response produces resistance or cure and a second type produces susceptibility and disease exacerbation—has been applied to infections with other pathogens in many hosts. However, whether this concept applies to VL is unclear. In experimental murine VL, both types of responses develop,185,256 and it is the balance of the Th1/Th2 responses that is considered to be important in controlling parasite replication, disease progression, or a cure. The balance between the two types of cellular immune responses also appears to be important in natural canine leishmaniosis and human VL.31,296 Understanding the profile of cytokines expressed in canine leishmaniosis is complex because of the limited number of studies reported, the broad spectrum of clinical disease stages investigated, the different tissues analyzed, the numerous methods used for evaluation of cytokines, the different timing during infection evaluated, and the differences between experimental and natural infections. Therefore, studies concerning the production of different cytokines in canine leishmaniosis are difficult to compare and interpret.31,296 L. infantum infection in dogs appears to induce a mixed Th1/Th2 response in both subclinical and sick dogs.31,296 Protective immunity to leishmaniosis in dogs is mediated by T cells.309 Based on in vitro and in vivo studies, it is widely accepted that macrophages play a central role in the control of Leishmania infection. Cytokines such as IFN-γ, IL-2, and tumor necrosis factor-α secreted by activated T cells induce canine macrophage antileishmanial activity.31 Nitric oxide produced by canine macrophages has been found to be the principal effector molecule mediating intracellular killing of Leishmania amastigotes by apoptotic cell death controlled by proteasome inhibitors.177 In addition, L. infantum–infected macrophages are lysed in a histocompatibility complex-restricted manner by CD8+ cytotoxic T cells, CD4+ cytotoxic T cells, or both. These processes are decreased or suppressed in sick dogs, in which T-cell proliferation with leishmanial antigen and antigen-specific IFN-γ production are depressed and a marked IgG response to the parasite ensues.100,240,309,310 A strong delayed-type hypersensitivity response with the leishmanin intradermal skin test indicative of a cell-mediated response to Leishmania infection is found in resistant dogs that have been exposed to the parasite and is absent in severely ill dogs.309,380 The cellular basis and mechanisms for the development of T-cell unresponsiveness in canine leishmaniosis are not understood fully. The majority of infected dogs are likely to develop positive specific cell-mediated immunity expressed as proliferation of lymphocytes stimulated in vitro by Leishmania antigen or in vivo by a positive skin test early in infection. However, as the disease progresses in susceptible dogs, these responses diminish.31 In addition, intracellular killing of the parasite by neutrophils and monocytes is impaired.59 The populations of lymphocytes and other leukocytes are altered during disease. Flow cytometry analysis of peripheral blood mononuclear cells and splenocytes has demonstrated that severe disease and high parasitism are accompanied by decreased numbers of CD5+ T, CD4+ T, CD8+ T, and CD 21+ B lymphocytes and monocytes. In contrast, increased levels of CD8+ T lymphocytes are a major feature of subclinical infection and low parasitism.167,326,326 Susceptibility and resistance to canine leishmaniosis appear to have a genetic basis. Candidate genes analyses have demonstrated association with susceptibility to canine leishmaniosis. A study on the polymorphism of the Slc11c1 (Solute carrier family 11 member a1) canine gene (former NRAMP1 gene), which encodes an iron transporter protein involved in the control of intraphagosomal replication of parasites and macrophage activation, has implied that susceptible dogs have polymorphism and mutations in this gene.11 Moreover, single nucleotide polymorphism caused by mutations in the promoter region of the Slc11a1 gene was associated with susceptibility to canine leishmaniosis and one haplotype (TAG-8-141) was associated with boxers’ predisposition to the disease.345 Similar mutations have also been described in humans.260 Results of a study showed that three of the 24 polymorphisms found in the Slc11a1 gene were associated with increased risk of clinical canine leishmaniosis.346 In contrast, differences in expression of Slc11a1 messenger RNA (mRNA) levels in circulating blood cells were not found between phenotypically resistant and susceptible dogs.60 A DLA class II DLA-DRB1 genotype, which is a dog major histocompatibility complex class II allele, has been linked to the risk of susceptibility to leishmaniosis in an endemic area in Brazil.321 Canine leishmaniosis is a chronic disease, and clinical signs of disease may develop 3 months to 7 years after infection. T-lymphocyte regions in the lymphoid organs become depleted, and antibody-producing B-cell regions proliferate. The proliferation of B lymphocytes, plasma cells, histiocytes, and macrophages results in generalized lymphadenomegaly, splenomegaly, and hyperglobulinemia. As with other chronic persistent intracellular infections, the immunoglobulin response is usually excessive but not protective and can eventually be detrimental. Autoantibodies in dogs with clinical leishmaniosis211,374 are thought to be associated with the development of pathologic phenomena such as immune-mediated thrombocytopenia.78,396 Serum anti-histone antibodies have been associated with glomerulonephritis in dogs with leishmaniosis.151 Another potential hazard of impaired T-cell regulation with exuberant B-cell activity is the generation of large amounts of circulating immune complexes (CICs).210 CIC deposition in the walls of blood vessels may cause vasculitis, polyarthritis, uveitis, and glomerulonephritis. In dogs, CIC deposition in the kidneys eventually results in renal failure, which is the main cause of death of dogs with leishmaniosis. Immune complexes that activate the complement cascade also induce vasculitis. This important pathologic mechanism is responsible for tissue necrosis and accountable for dermal, visceral, and ocular lesions found in this disease. Systemic vasculitis may lead to local ischemia that causes visceral and cutaneous necrosis and, although rarely, to nervous system involvement.144,319,319 CICs may also include cryoglobulins. These proteins may precipitate in the blood vessels of the extremities when exposed to cold and cause ischemic necrosis.371 Canine leishmaniosis is most often associated with various skin lesions, which are frequently generalized rather than local because L. infantum disseminates all over the body.318 In addition, microscopic lesions and presence of parasite is detected in normal-appearing skin from sick dogs and not only in dermal lesions.376 Extracellular matrix assessment in the skin of sick dogs demonstrated a decrease in collagen type I and an increase in collagen type III fibers proportional to the severity of the skin lesion and tissue destruction.155 Ocular lesions include anterior uveitis, conjunctivitis, keratoconjunctivitis sicca, blepharitis, or a combination of these.302,303 In keratoconjunctivitis sicca, inflammatory infiltrates located around the lacrimal ducts cause secretory retention and a decrease in tear production.271 Muscle weakness is observed in canine leishmaniosis and is associated with mononuclear myositis, neutrophilic vasculitis and IgG immune complexes in muscle tissues in conjunction with serum anti-myofiber antibodies.290,407 Dogs with leishmaniosis may show signs of a hemorrhagic diathesis, manifested primarily as epistaxis, and less commonly as hematuria and hemorrhagic diarrhea. Epistaxis appears to be the result of multiple and variable pathogenetic factors involved in primary and secondary hemostasis, such as thrombocytopathy, hyperglobulinemia-induced serum hyperviscosity, and lymphoplasmacytic or granulomatous rhinitis with or without nasal mucosa ulceration.70,184,184 Anemia usually develops as a sequel to the decreased erythropoiesis of chronic disease or chronic kidney disease but may be aggravated by blood loss. Canine leishmaniosis is a disease in which infection does not equal clinical illness because of the high prevalence of subclinical infection.31,381 It may be manifested as a subclinical infection, a self-limiting disease, or the classical non-self-limiting and severe illness.378 Canine leishmaniosis due to L. infantum is often classified as visceral disease in compliance with the classification of the disease caused by this pathogen in humans; however, dogs usually have both visceral and cutaneous involvement. Canine leishmaniosis is essentially a chronic systemic disease that may potentially involve any organ, tissue, and biological fluid and is manifested by a plethora of clinical signs.378 The main clinical findings found on physical examination in typical canine leishmaniosis cases include skin lesions, local or generalized lymphadenomegaly, loss of body weight, exercise intolerance, decreased appetite, lethargy, splenomegaly, polyuria and polydipsia, ocular lesions, epistaxis, onychogryphosis, lameness, vomiting, and diarrhea (Table 73-1).31,69,192,378 TABLE 73-1 Clinical Findings in Dogs with Leishmaniosis69,192,303,370 The prevalence of cutaneous lesions in dogs with clinical leishmaniosis ranges between 56% to 90%.69,192,192 Dermatologic abnormalities may occur in the apparent absence of other obvious signs of disease, but any animal with dermal manifestations of leishmaniosis is likely to have visceral involvement, because the parasites disseminate throughout the body before generalized skin lesions develop. Dermatologic abnormalities vary in character and extent but are rarely pruritic. Several dermatologic entities have been described137,145,192,194: (1) exfoliative dermatitis with alopecia, which can be generalized or localized over the face, ears, and limbs (Fig. 73-5); (2) ulcerative dermatitis over bony prominences, and in mucocutaneous junctions, paws, and the ear pinnae (Fig. 73-6); (3), focal or multifocal nodular dermatitis; (4) mucocutaneous proliferative dermatitis (Fig. 73-7); and (5) papular dermatitis (Fig. 73-8).281 Lymphadenomegaly of multiple superficial lymph nodes frequently develops208 with lymph nodes enlarging to two to six times their normal size and occasionally mimicking clinical findings of lymphoma. Splenomegaly is also frequently detected by abdominal palpation. Approximately 16% to 80.5% of the dogs diagnosed with canine leishmaniosis have ocular lesions, including conjunctivitis, blepharitis, keratoconjunctivitis, and uveitis (Fig. 73-9).69,148,302,303,370 In some cases, ocular abnormalities are the only clinical signs.303 Abnormally long or brittle nails (onychogryphosis), a rather specific finding, develop in a small proportion of patients. Epistaxis is another clinical sign that may develop in conjunction with other typical abnormalities or as the only presenting sign in canine leishmaniosis (Fig. 73-10).184,306 Weight loss and muscle atrophy are the most common signs of visceral involvement (Fig. 73-11). Some dogs lose weight despite having a ravenous appetite. A serious worsening of condition is often associated with ensuing chronic kidney disease. A progressive chronic kidney disease may be accompanied by anorexia, mental depression, polyuria, polydipsia, and vomiting. Transient diarrhea may occur. Kidney disease may be the sole apparent abnormality in dogs with leishmaniosis; dogs with this condition in endemic areas should be tested for Leishmania infection. In cases of overt disease, decreased physical activity is obvious and related to somnolence, decreased endurance, and locomotion disturbances. Locomotion disturbances may be caused by neuralgia, erosive and non-erosive polyarthritis, polymyositis, footpad clefts, interdigital ulcers, osteoarticular and osteolytic lesions, or proliferative periostitis.6,48 Paraparesis was reported in one dog as a result of a granuloma formation in the vertebral canal.66a Body rectal temperature may fluctuate but is usually normal or subfebrile. The clinical picture may be complicated by conditions such as demodicosis, pyoderma, gastrointestinal disease, and pneumonia. Combined infections with Ehrlichia, Babesia, Hepatozoon, Trypanosoma, Bartonella, and Dirofilaria might lead to exacerbation of clinical signs and/or clinicopathologic abnormalities* when Leishmania infection occurs in regions where these organisms are also endemic. Other less common manifestations may include pericardial tamponade,142 masticatory myositis,407 pancreatitis,62 meninigitis,412 chronic colitis,129 pemphigus,153 and polyarthritis.388 Thrombosis has also occurred as a result of the nephrotic syndrome caused by glomerulonephritis.138,141 Signs caused by disseminated intravascular coagulation or its complications may occur.143 Diagnosis is usually performed to confirm disease in a dog with clinical signs or clinicopathologic abnormalities compatible with canine leishmaniosis. However, detection of infection may also be pursued for research studies, for screening clinically healthy dogs living in endemic regions, to prevent transmission by blood transfusion, to avoid importation of infected dogs to nonendemic countries, and to monitor response to treatment.258,378 Different diagnostic procedures and interpretations of test results might be used accordingly, depending on the purpose of the diagnostic investigation.378 Accurate diagnosis of clinical leishmaniosis often requires an integrated approach consisting of clinicopathologic tests and specific diagnostic assays.378 The specific assays include microscopic demonstration of parasites in cytologic preparations or histopathologic specimens, serology, culture of the organism in appropriate medium, or detection of parasite DNA using molecular methods. A summary of the clinicopathologic findings from three large case series studies on canine leishmaniosis69,193,193 is shown in Table 73-2. The most consistent serum biochemistry findings in dogs with clinical leishmaniosis are serum hyperproteinemia with hyperglobulinemia and hypoalbuminemia resulting in a decreased albumin/globulin ratio. Polyclonal beta and gamma hyperglobulinemia in dogs from L. infantum–endemic regions with no apparent cause or in dogs that have traveled to such areas should be investigated as a possible indication of leishmaniosis. Acute-phase proteins are elevated in serum of sick dogs.241 Mild increases of liver enzyme activities are frequent; however, grossly elevated liver enzyme activities, severe azotemia, or both are found in only a minority of dogs with leishmaniosis. Proteinuria and some renal abnormalities develop in most dogs with this disease, and subsequent kidney disease caused by immune-complex glomerulonephritis eventually develops in dogs with progressive pathologic renal dysfunction. The urinary protein/creatinine ratio and enzymuria have been proposed as tests for assessing renal damage in affected animals.292,293 TABLE 73-2 Clinicopathologic Abnormalities in Dogs with Leishmaniosis69,193,193 Mild to moderate nonregenerative anemia is frequently found. Mild leukocytosis, leukopenia, and pancytopenia are inconsistent findings.306 However, lymphopenia is frequently reported in dogs with leishmaniosis. Serum hyperviscosity, thrombocytopathy,306 secondary immune-mediated thrombocytopenia,77,396 impaired secondary hemostasis, and fibrinolysis70 may also be detected. Test results for antinuclear antibodies can be positive in dogs with L. infantum infections, especially if copathogenic infections are present.374 Parasites are rarely detected in peripheral blood smears.137 Lymphocytic and neutrophilic inflammation is observed with Leishmania amastigotes in the synovial fluid in dogs with both erosive and nonerosive polyarthritis.6 Dogs with neurologic signs have most commonly a lymphocytic pleocytosis.207 A reduction in semen quality of dogs with leishmaniosis has been reported with poor progressive motility and large score of total sperm defects and detached normal heads. Semen quality is partially reverted after long-term treatment.26 Various serologic methods have been used to detect serum anti-Leishmania antibodies. Methods have included indirect fluorescent antibody testing, the enzyme-linked immunosorbent assay (ELISA), immunochromatography with rapid in-house devices, direct agglutination assays, and Western blotting.* In general, most of these methods have good sensitivities and specificities for the diagnosis of clinical leishmaniosis. However, sensitivity and specificity greatly depend on the antigens employed. Whole-parasite extracts are sensitive for the detection of subclinical or clinical canine infections but provide a somewhat lower specificity.124 On the other hand, assays that include recombinant protein antigens are very specific but may lack sensitivity for the detection of clinically healthy infected dogs, depending on the antigen employed.316 The recombinant proteins reported to be highly sensitive for detection of infection in dogs are K39, K26, KMP11, SMT,157,399 cysteine proteinase,311 A2,65 and the chimeric protein Q.385 Another study demonstrated the high sensitivity and specificity of a ribosomal protein extract.71 A diagnostic algorithm summarizing the use of antibody titers for leishmaniasis is presented in Fig. 73-12. High antibody levels are associated in canine leishmaniosis with high tissue parasite loads and disease.229,328 Anti-Leishmania antibodies are rarely undetectable in infected dogs with clinical signs of leishmaniosis. Therefore, a high positive antibody titer strongly supports the diagnosis and is conclusive of leishmaniosis in dogs with compatible clinical signs or clinicopathologic abnormalities.378 In cases with low antibody titers and compatible clinical signs, additional detection methods are advised to exclude or confirm the disease because a low positive titer result may be detected in dogs that are subclinical carriers (see Fig. 73-12).378 Cross-reactivity with different pathogens is possible with some serologic tests, especially those based on whole-parasite antigen. Cross-reactions are less likely to occur when using recombinant peptides or extracts311,316 or ribosomal protein extracts.71 Cross-reactivity has been reported with other species of Leishmania124,316 and Trypanosoma.33 In contrast, cross-reactivity is less likely to occur against Babesia canis and Ehrlichia canis.280 Diagnosis can be based on cytologic or histologic identification of amastigotes, either contained in macrophages or free in routinely stained smears. Organisms are often found in lymph nodes, splenic aspirates, skin touch impressions, bone marrow, or other tissues and body fluids. The specificity of these methods is virtually 100%, but depending on the time spent searching for parasites, the maximum sensitivity is plus or minus 80% in dogs with clinical signs of the disease and lower in subclinically infected seropositive dogs. Cytologic studies may reveal few or no demonstrable parasites in dogs with overt clinical signs of disease. Identification of amastigotes in formalin-fixed, paraffin-embedded sections of canine skin or visceral tissues may be facilitated by immunohistochemical methods such as immunoperoxidase staining (Fig. 73-13).58,130 The diagnosis may also be established by the culture of parasites from tissues in Novy-MacNeal-Nicolle medium or Schneider’s Drosophila medium or by the inoculation of hamsters. PCR demonstration of leishmanial DNA in tissues of infected animals is sensitive. It is routinely used for diagnostic purposes, research studies, and screening for canine blood donors.23,244 The small-subunit ribosomal RNA (rRNA) gene, the internal transcribed spacer of the ribosomal operon, and high-copy sequences of kinetoplast DNA (kDNA) have been used as PCR targets. The kDNA assays are considered the most sensitive due to the high copy number of this target.196 PCR can be performed on DNA extracted from tissues, blood, body fluids, or even histopathologic specimens.180,341 PCR on bone marrow, lymph node, spleen, or skin is most sensitive and specific for the diagnosis of leishmaniosis.221,228 PCR on whole blood, buffy coat, and urine is less sensitive than the aforementioned tissues.228,383 Noninvasive conjunctival PCR has been shown to be accurate in the diagnosis of seropositive dogs with clinical leishmaniosis.125,201a,389 Severely affected patients are usually cachectic and suffer from muscle atrophy. The skin and hemolymphatic organs are primarily affected. Generalized lymphadenomegaly and splenomegaly are usually present. Hepatomegaly may be present, but it is less common. Small, light-colored focal nodular granulomas may develop in various organs, including the skin and the kidneys. Mucosal ulcerations in the nasal cavity, stomach, intestine, and colon are occasionally observed. Petechiae and ecchymotic bleeding in mucosal and serosal membranes develops in some cases. Osteolytic or proliferative periosteal lesions may be found in various parts of the skeleton.6 The typical histopathologic finding in the majority of affected tissues is an inflammatory reaction associated with macrophages in the presence or apparent absence of Leishmania amastigotes. Amastigote numbers may vary from very few organisms within macrophages to large numbers in rarer events. Lymphoplasmacytic inflammation is also common in dogs with leishmaniosis. Classical histopathologic lesions have been described mainly in organs with abundant mononuclear-phagocytic system cells such spleen, lymph nodes, bone marrow, liver, gastrointestinal tract,4 and skin. Moreover, other organs such as the eyes302 and nasal cavity306 may also develop similar inflammatory patterns. A reactive lymphoid hyperplasia is found in lymphoid organs including the lymph nodes and spleen in association with monocytic hyperplasia in the spleen and bone marrow38,154,154 and variable numbers of Leishmania amastigotes. The most common cutaneous histopathologic findings are periadnexal nodular to diffuse pyogranulomatous or granulomatous dermatitis, together with orthokeratotic to parakeratotic hyperkeratosis, acanthosis, crusting, and ulceration. Other histopathologic patterns, such as subcorneal pustular dermatitis, lichenoid dermatitis, vasculitis, and panniculitis, have also been described.48,194,376,378 Renal lesions include glomerulonephritis, tubulointerstitial nephritis, and rarely amyloidosis.79,272,272 Membranoproliferative glomerulonephritis is more frequently associated with chronic kidney disease. Mesangioproliferative lesions and minimal-change glomerulonephritis have been detected in kidney tissue from infected dogs without clinicopathologic evidence of renal disease.313 In the liver, mild changes have been observed with the presence of individual or clustered macrophages, mostly within the sinusoids.323 Moderate changes, the most common finding, are characterized by mononuclear infiltration in the portal space and hepatic parenchyma.251,323 Hepatic granulomas and collagenogenesis (intralobular fibrosis) have been reported as a common finding in canine leishmaniosis.250,251 More severe hepatic pathology is characterized by marked lymphoplasmacytic portal hepatitis with occasional infiltration to the parenchyma and portal fibrosis.323 Histopathologic changes in the eyes are observed, in order of frequency, in the conjunctiva and limbus, ciliary body, iris, cornea, sclera and iridocorneal angle, chorid, and optic nerve sheath, with different inflammatory patterns and variable numbers of Leishmania organisms.302 Pathologic changes in muscle are characterized by scattered to diffuse mononuclear myositis, myonecrosis, and endomisial fibrosis.290 In the central nervous system, inflammatory cell infiltration including lymphocytes, macrophages, plasma cells, and some neutrophils was reported in the choroid plexi, subventricular zone, and leptomeninges as well as around parenchymal blood vessel in dogs with high anti-Leishmania antibody titers and clinical leishmaniosis with or without neurologic signs.252 Cerebral blood vessel walls presented positive labeling against anti-Leishmania antibody in a histopathologic study, suggesting the deposition of circulating parasite antigens in the central nervous system.252 No gross lesions are normally detected in the external or internal genital organs of canine males and bitches.111,367 However, a high frequency of microscopic inflammatory lesions and presence of Leishmania amastigotes have been observed in studies on the male genital organs mainly in the epididymis, glans penis, prepuce, and testis.111 In contrast, the only histologic change found in bitches was a mild to moderate vulvar dermatitis with no changes in other genital organs.367 Canine leishmaniosis is more resistant to therapy than human leishmaniosis, and only rarely are Leishmania organisms completely eliminated with available drugs.32,276 Relapses necessitating retreatment are the rule rather than the exception, although dogs frequently become cured of the clinical disease. For a summary of the drug dosages, consult Table 73-3 and the discussion later. Follow-up and monitoring of clinical and laboratory parameters of treated dogs are essential for adequate management.220a,299b,378 TABLE 73-3 Antimicrobial Therapy for Canine Leishmaniosis PO, By mouth; SC, subcutaneous. aFor additional information on use of these drugs, see the Drug Formulary in the Appendix. bDose per administration at specified interval. cSome long-term survivors must be treated on multiple occasions because of relapses. dSome studies suggest combined therapy with a 4- to 8-week course of meglumine antimoniate in combination with allopurinol. This is followed by allopurinol alone for at least 6–12 months and is currently considered the most effective treatment of L. infantum infections. eCan be combined with allopurinol as an alternative to the meglumine antimoniate. In such cases, treatment with allopurinol is continued for at least 6–12 months after miltefosine discontinuation. For decades, pentavalent antimonials have been the primary drug for treatment of canine and human leishmaniosis. They selectively inhibit the protozoal enzymes required for glycolytic and fatty acid oxidation. Meglumine antimoniate (see the Drug Formulary in the Appendix) is the main antimonial used for treatment of dogs. It is injected subcutaneously at 75 to 100 mg/kg/day for 4 to 8 weeks and may cause cutaneous cellulitis or abscesses at the injection site and be potentially nephrotoxic.179,332,372,395,406 The development of L. infantum strains that are resistant to pentavalent antimonials has been reported in France, Spain, and Italy and is a veterinary and public health concern.63,64,159,199 Meglumine antimoniate was used in the treatment of a pregnant bitch, and two pups that survived were followed until 1 year of age without evidence of developing leishmaniasis.387a Allopurinol has become an indispensable part of the treatment of canine leishmaniosis, frequently used in combination with other drugs.* It is a hypoxanthine compound that is metabolized by Leishmania spp. to produce an analogue of inosine. The analogue is incorporated into leishmanial RNA, causing faulty protein translation and inhibition of parasite multiplication. It is administered orally, has few adverse effects, and is readily available, even in the United States and countries where meglumine antimoniate is not marketed (see the Drug Formulary in the Appendix).67,193 A dose of 10 mg/kg twice daily often results in remarkable clinical improvement within 4 weeks and reduction of parasites to undetectable numbers. However, relapses frequently occur once therapy has been discontinued. Even after conscientious administration of the drug for 6 months, complete recovery is rare,205 and deterioration of kidney function may continue during allopurinol therapy despite resolution of dermal lesions and general improvement in the clinical condition. Use of allopurinol causes hyperxanthinuria, which may occasionally produce urolithiasis. Combined treatment with meglumine antimoniate and allopurinol is considered as the most effective therapy and constitutes the most frequently used protocol against the disease.258,378 The combination is administered for 4 to 8 weeks, followed by continuation with allopurinol alone for at least 6 to 12 months. Miltefosine (see the Drug Formulary in the Appendix) is an alkylphospholipid with a direct toxic effect on Leishmania parasites that has proven to be effective for the treatment of human VL and has been introduced for use in veterinary medicine.226,243 It is administered orally to dogs at 2 mg/kg/day for 4 weeks and is an alternative to meglumine antimoniate when combined with long-term allopurinol therapy.227,259 Amphotericin B (AMB), a polyene macrolide primarily used as an antifungal drug, also has activity against some protozoa. It acts by binding to ergosterol and altering the cell membrane permeability. AMB has a good efficacy against canine leishmaniosis,75,197,197 but its use is limited because it is administered intravenously and has a profound toxic effect on the canine kidney by causing renal vasoconstriction and reduction of the glomerular filtration rate and possibly by acting directly on renal epithelial cells. Liposomal AMB is effective in the treatment of humans and has largely replaced therapy of human patients with antimonials in Italy and other European countries.159 However, a study on the use of liposomal AMB in dogs failed to show that treated dogs had long-term clinical improvements with elimination of the infection.278 Additional drugs that are not recommended as first-line therapy for canine leishmaniosis because of the adverse effects associated with treatment include pentamidine and aminosidine.315,331 Other drugs that have been evaluated for antileishmanial efficacy in dogs include ketoconazole, metronidazole and spiramycin, and marbofloxacin.258 In a trial comparing the efficacy of conventional treatment with meglumine antimoniate and allopurinol against use of metronidazole and spiramycin, treated dogs showed some clinical improvement without a parasitologic cure, similar to control dogs.304 If dogs are seriously ill, and especially if they are in severe renal failure, it may be necessary to restore fluid and acid-base balances before antileishmanial drugs are administered. The prognosis of canine leishmaniosis depends on the severity of injury to the dog’s systems at the time of diagnosis and the dog’s individual response and rate of deterioration. In dogs that have not reached a progressive state of renal failure, treatment frequently significantly improves dermal and visceral signs of the disease. Immune responses were monitored in dogs before and after treatment and have indicated that in some cases, parasite-specific cell-mediated immunity that is absent before therapy is regained after drug administration but may deteriorate again during a clinical relapse.103,267,267
Leishmaniases
Global Aspects of Leishmaniosis
Etiology
Epidemiology
Canine Leishmaniosis
Epidemiology
Pathogenesis
Clinical Findings
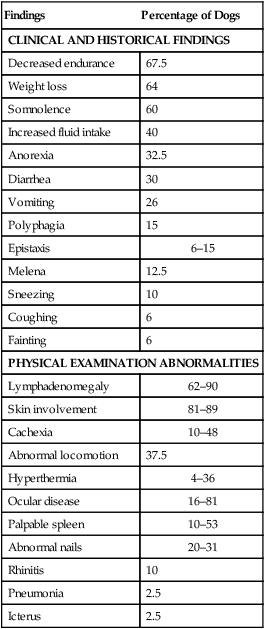
Findings
Percentage of Dogs
CLINICAL AND HISTORICAL FINDINGS
Decreased endurance
67.5
Weight loss
64
Somnolence
60
Increased fluid intake
40
Anorexia
32.5
Diarrhea
30
Vomiting
26
Polyphagia
15
Epistaxis
6–15
Melena
12.5
Sneezing
10
Coughing
6
Fainting
6
PHYSICAL EXAMINATION ABNORMALITIES
Lymphadenomegaly
62–90
Skin involvement
81–89
Cachexia
10–48
Abnormal locomotion
37.5
Hyperthermia
4–36
Ocular disease
16–81
Palpable spleen
10–53
Abnormal nails
20–31
Rhinitis
10
Pneumonia
2.5
Icterus
2.5
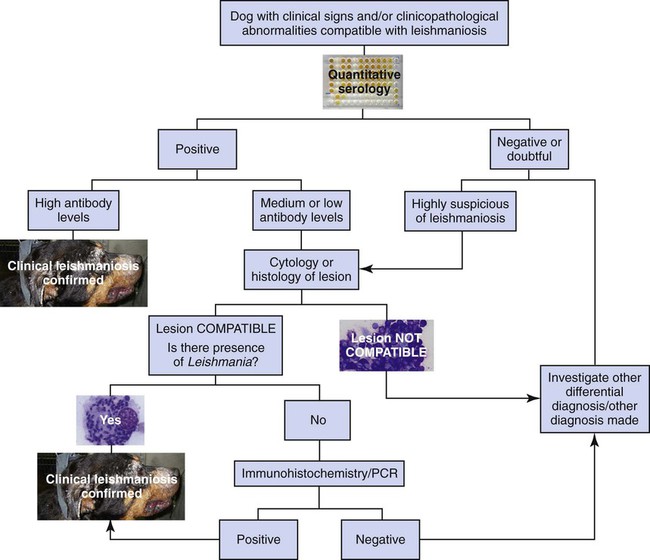
Diagnosis
Clinical Laboratory Findings
Abnormality
Percentage of Dogs
Hyperproteinemia
63.3–72.869,193
Hyperglobulinemia
76–10069,370
Hypoalbuminemia
68–9469,370
Decreased albumin/globulin ratio
7669
Azotemia
16–4569,370
Increased serum alkaline phosphatase activity
16–5169,370
Increased alanine aminotransferase activity
16–6169,370
Proteinuria
71.5–85193,370
Anemia
60–73.4193,370
Leukocytosis
2469
Leukopenia
22370
Thrombocytopenia
29.3–5069,370
Positive for antinuclear antibody
31–5369,370
Positive Coombs’ test
21–8469,370
Antibody Testing
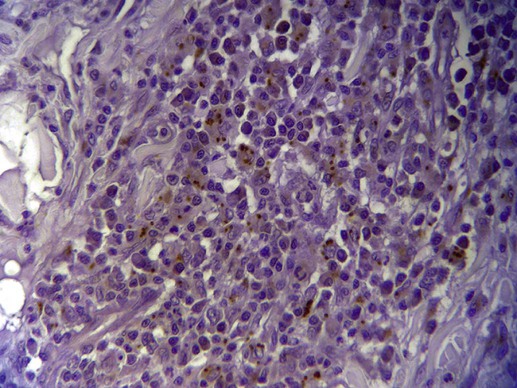
Organism Identification by Microscopy and Culture
Polymerase Chain Reaction
Pathologic Findings
Therapy
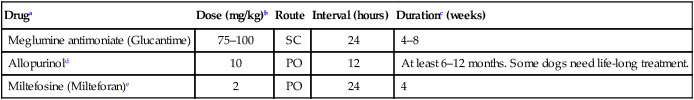
Druga
Dose (mg/kg)b
Route
Interval (hours)
Durationc (weeks)
Meglumine antimoniate (Glucantime)
75–100
SC
24
4–8
Allopurinold
10
PO
12
At least 6–12 months. Some dogs need life-long treatment.
Miltefosine (Milteforan)e
2
PO
24
4
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine