27 Laminitis
Laminitis may best be regarded as a syndrome rather than a single disease entity because there are multiple inciting causes and possibly pathophysiological pathways (Harris 2011), which can be summarized into three primary categories (Table 27-1): (1) sepsis/systemic inflammatory conditions (e.g., gastrointestinal disease, septic metritis, pneumonia, carbohydrate overload); (2) endocrine/metabolic (e.g., associated with insulin resistance, obesity &/or pituitary pars intermedia dysfunction); and (3) mechanical overload (supporting limb laminitis). Nutrition has long been linked to laminitis. Indeed, around 350 BCE Aristotle used the term “barley disease” in obvious reference to the development of laminitis after consumption of excessive cereal grains (grain overload) and, since the 1970s, carbohydrate overload (the bolus administration of large quantities of a starch gruel) has been one of the primary experimental models of laminitis (Harris & Geor 2010). Over the last 20 years, survey studies have indicated that many cases of laminitis occur in horses and ponies kept at pasture, giving rise to the term pasture-associated laminitis (Hinckley & Henderson 1996, USDA 2000, Treiber et al 2006, Menzies-Gow et al 2010a).
Table 27-1 Conditions Associated with an Increased Risk of Laminitis (Eades 2010, Harris & Geor 2010)
Clinical cases of pasture laminitis often occur under conditions that favor accumulation of rapidly fermentable nonstructural carbohydrates (fructans, simple sugars and/or starches) in grass and clover. It has been argued, therefore, that pasture laminitis is triggered by carbohydrate overload of the hindgut and the systemic absorption of substances that initiate lamellar failure (Bailey et al 2004a). This hypothesis is supported by experimental studies in which the administration of oligofructose, a commercial fructan, induces laminitis in healthy horses (van Eps and Pollitt 2006). However, the extent to which this model reflects events during development of naturally-occurring laminitis is unknown, particularly in view of the fact that only a very small proportion of any population develops pasture laminitis even during periods of theoretical highest risk. This observation has stimulated research on possible reasons for the apparent increased susceptibility of certain horses and ponies. Relatively recently, evidence has emerged that animals with an insulin resistant phenotype are at an increased risk for pasture-associated laminitis (Treiber et al 2005, 2006, Bailey et al 2008, Carter et al 2009). Additionally, the discovery that prolonged intravenous infusion of insulin induces laminitis in healthy animals has widened the perspective on potential pathophysiologic mechanisms of laminitis (Asplin et al 2007, De Laat et al 2010) and raised the possibility that diet (including pasture grazing) might provoke episodes of laminitis via effects on insulin dynamics, particularly in insulin resistant animals. The term equine metabolic syndrome (EMS) is currently used to describe horses and ponies with an insulin-resistant phenotype linked with laminitis susceptibility (Geor & Frank 2009, Frank et al 2010), while endocrinopathic laminitis is sometimes used to describe the EMS-associated laminitis as well as that associated with pituitary pars intermedia dysfunction (PPID, equine Cushing’s disease; McGowan 2010).
Epidemiology and risk factors
Recent systematic reviews have highlighted the lack of quality information with respect to the epidemiology of equine laminitis, including data on prevalence, inciting causes and risk factors (Wylie et al 2011a,b). The reported disease frequency has ranged from 1.5% to 34% in different studies, with factors such as sample size and type (e.g. ponies vs. horses), underlying laminitis etiology, case definition, modes of diagnosis, climate, and feeding/management practices likely contributing to this wide variation in frequency of laminitis (Wylie et al. 20011a). Hinckley and Henderson (1996) surveyed veterinarians and horse owners to estimate the number of acute and chronic laminitis cases in a population of approximately 113 000 horses and reported about 1700 cases of both acute and chronic laminitis with an overall prevalence of 3%. Approximately 61% of the laminitis cases occurred in horses kept at pasture (Hinckley & Henderson 1996). The United States Department of Agriculture (USDA) survey reported that 13% of horse operations had had at least one case of laminitis during the previous 12 months with 1% of horses affected at any one time (based on owner responses). Laminitis was identified as the most common cause of foot lameness, accounting for up to 16% of all lameness cases. Additionally, nutritionally-associated laminitis accounted for more than 50% of the cases, with 46% attributed to pasture grazing and 7% to grain overload (Kane et al 2000, USDA 2000).
A retrospective study in the south of England reported the prevalence, incidence and seasonality of laminitis in a population of about 1000 horses/ponies kept on a single farm and maintained at pasture (Menzies-Gow et al 2010a). Over a 6-year period, 23.5% of the population had at least one episode of veterinary diagnosed laminitis; the highest prevalence (2.6%) and incidence (16 cases/1000 animals) of laminitis occurred in May. A positive association was found between hours of sunshine and the prevalence and incidence of laminitis, but there were no associations with rainfall or monthly temperature. The association between hours of sunshine and incident laminitis was presumed to reflect altered nutritional intake (i.e., increased consumption of nonstructural carbohydrates during periods of bright sunshine that promote plant photosynthesis and carbohydrate accumulation) rather than the direct effect of exposure of horses to sunlight. Other studies have reported an increased risk of laminitis during the spring and summer months (Dorn et al 1975, Hinckley & Henderson 1996, USDA 2000) although evidence of seasonality is inconsistent (Polzer & Slater 1997, Wyllie et al 2011b).
Menzies-Gow et al (2010a) also observed that approximately one-third of animals diagnosed with laminitis had at least one more episode during the study period. Moreover, about 24% of these animals had a repeated episode in the same year as the original diagnosis. These observations confirm the clinical impression that some animals are prone to repeated episodes of laminitis (Buckley et al 2007) and focus attention on the possibility that there are phenotypic or genetic factors associated with susceptibility. With regards to genetic factors, laminitis in a foal attributable to disruption of a single molecule of the hemidesmosome adhesion complex was believed to be the result of an inherited recessive defect that lead to failure in expression of the protein plectin (French & Pollitt 2004). Additionally, Belgian foals with mechanobullous disease (epidermolysis bullosa) are at an increased risk of laminitis (Frame et al 1988).
In the study by Menzies-Gow et al (2010a), univariate analysis revealed that animals ≥14.3 hands in height were at reduced risk of laminitis while females, light animals (<400 kg) and animals with a weight-to-height ratio <7.51 kg/in were at higher risk. Female gender also was a significant risk factor in multivariate analysis, a finding in agreement with several other studies (Dorn et al 1975, Alford et al 2001, Slater et al 1995). The reason for the apparent increased laminitis risk in mares is not known. The height and weight associations with laminitis lend support to the clinical impression that ponies are at higher risk as compared to horses, although in multivariate analysis weight but not height was revealed as a significant risk factor (Menzies-Gow et al 2010a). In a prospective study of pasture-associated laminitis, the majority of affected animals (89 of 107 cases) were overweight/obese (Menzies-Gow et al 2010b). Additionally, overweight animals were at increased risk of severe clinical signs and were less likely to survive. These findings are consistent with earlier reports that identified obesity as a risk factor for laminitis (Alford et al 2001). Increased load bearing by the feet is one potential explanation for the increased risk of laminitis in overweight/obese animals.
Several studies have characterized metabolic risk factors for pasture-associated laminitis in ponies (Treiber et al 2005, 2006, Bailey et al 2007, 2008, Carter et al 2009). In an inbred herd of Welsh and Dartmoor ponies, laminitis risk was associated with the clustering of hyperinsulinemia, obesity and hypertriglyceridemia – this phenotype was termed “prelaminitic metabolic syndrome (PLMS)” (Treiber et al 2006). The PLMS criteria predicted 11 of 13 cases of clinical laminitis observed in May of the same year, with an odds ratio of 10.4 (i.e. ponies with this phenotype were at approximately 10-times higher risk for laminitis). Pedigree analysis suggested a dominant mode of inheritance of the PLMS phenotype, supporting the possibility of a genetic basis for laminitis predisposition (Treiber et al 2006). A subsequent study of this pony population demonstrated that the presence of obesity (generalized or regional, i.e., cresty neck), hyperinsulinemia (insulin >32 mU/l when sampled on winter pasture) or hyperleptinemia (>7.3 ng/ml) were useful predictors of laminitis episodes when ponies were exposed to high carbohydrate pasture (Carter et al 2009). A study of out-bred ponies in the UK also revealed an association between apparent insulin resistance (IR) and predisposition to pasture laminitis, and provided evidence of hypertension in the high risk ponies (Bailey et al 2008). Interestingly, expression of this metabolic phenotype was only noted in summer but not winter, suggesting interaction with environmental factors such as consumption of summer pasture forage (Bailey et al 2008). As mentioned, EMS rather than PLMS has been broadly adopted to describe horses and ponies with an insulin resistant phenotype and associated predisposition to laminitis. These animals are often overweight or obese (or have localized large fat deposits, e.g., a “cresty neck”) and exhibit abnormal insulin dynamics, either resting hyperinsulinemia or an exaggerated increase in insulin concentration in response to oral or intravenous glucose challenges (Frank et al 2010, Frank 2011). In addition to pony breeds, Morgan Horses, domesticated Spanish mustangs, European warmbloods and American saddlebreds are thought to be at increased risk for development of EMS; however, currently there is insufficient evidence to support or refute this belief. It should be emphasized that non-obese animals that may or may not show IR also can experience recurrent laminitis.
Laminitis is a common occurrence in horses with PPID (equine Cushing’s disease), with prevalence between 50% and 80% in some reports (Schott et al 2001, Donaldson et al 2002, 2004, McGowan 2010). Donaldson et al (2004) reported that 28 of 40 cases of laminitis (70%) seen in primary veterinary practice were associated with high plasma adrenocorticotropic hormone (ACTH) concentrations and a presumptive diagnosis of PPID.
Key Points –
Risk factors for laminitis
• Pasture turnout can be a trigger factor for laminitis, even in lean, non-obese animals that do not have increased basal plasma insulin concentrations
• Horses and ponies with an obese, insulin-resistant phenotype are at an increased risk of laminitis when turned out to pasture with high nonstructural carbohydrate content (simple sugars, fructans, and starch)
• Animals with pituitary pars intermedia dysfunction (PPID) are at increased risk of laminitis
• Predisposing events and risk factors for laminitis may be additive in nature.
Pathogenesis
Most of our current knowledge of the pathogenesis of laminitis has been derived from four experimental models of the condition, specifically: (1) carbohydrate overload (high starch); (2) black walnut extract administration; (3) oligofructose (fructan) overload; and (4) insulin-induced laminitis (Eades 2010). Findings from studies using these models have given rise to several interlinking hypotheses regarding key mechanisms of lamellar failure and laminitis development, including inflammation, activation of degradative matrix metalloproteinases, hemodynamic alterations within the digital microvasculature, and disruption of lamellar epithelial cell function by hyperinsulinemia (Belknap et al 2009, Harris & Geor 2010, Eades, 2010). Although much progress has been made in describing cellular and molecular events in lamellar tissue during the development of laminitis, understanding of the pathophysiology of the condition remains incomplete. Moreover, as mentioned, questions remain regarding the relevance of these experimental models to the pathogenesis of naturally-occurring laminitis. The following subsections briefly discuss these models in the context of nutritionally associated and endocrinopathic laminitis.
Carbohydrate overload
Alimentary carbohydrate overload and associated risk for development of laminitis can occur when horses and ponies ingest excessive quantities of simple sugars, starches and/or fructans. Circumstances for carbohydrate overload include the ingestion of large amounts of starch-rich cereal grains (accidental access or inappropriate feeding management) or pasture forage that, under certain conditions, may have high content of these nonstructural carbohydrate (NSC) fractions (especially fructans). Experimentally, carbohydrate overload-induced laminitis has been created by the bolus administration (via gastric gavage) of a mixture of cornstarch and wood flour (~17 g/kg bodyweight (BW); Obel 1948, Garner et al 1975, Pollitt & Visser 2010) or oligofructose (OF, 5.0–12.5 g/kg BW; van Eps & Pollitt 2006, Kalck et al 2009). It is thought that most of the consequences of carbohydrate overload occur after passage of undigested starch or fructan to the hindgut and stem from the rapid fermentation of this substrate within the cecum and large intestine. Horses have a limited capacity for prececal digestion of starch; the exact limit varies between horses and is also affected by grain type and meal size but in general there is some “by-pass” of undigested starch to the hindgut especially when more than 2 g starch/kg BW is consumed as a single meal or unprocessed corn/barley is fed (see Chapter 8). Fructans are not degraded by mammalian enzymes (Nilsson et al 1988). Some ingested fructan (in particular the short chain inulin-type fructans) may undergo acid hydrolysis in the stomach or microbial fermentation in the foregut (Coenen et al 2005); however the bulk of the ingested material will pass unaltered to the large intestine where it will undergo fermentation (Longland et al 2012a).
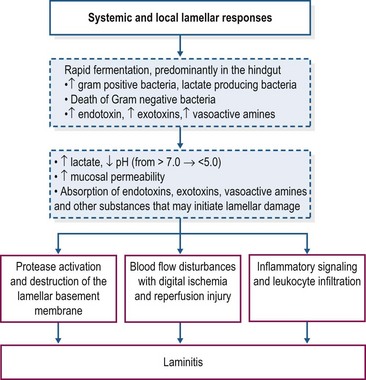
Carbohydrate overload with starch or oligofructose elicits similar digestive and systemic disturbances. The possible sequence of events that leads to the development of laminitis is shown in Fig. 27.1. The rapid fermentation of starch or oligofructose in the hindgut induces profound changes in the hindgut microbiome, with disappearance of Escherichia coli and rapid proliferation of Gram-positive bacteria that preferentially ferment these substrates and produce lactic acid as an end-product of fermentation, particularly the equine hindgut streptococcal species (EHSS; Streptococcus bovis and Streptococcus lutetiensis; Milinovich et al 2006, 2010). D– and L-lactate are produced by bacterial fermentation, contributing to a sharp increase in hindgut acidity; the pH of intestinal contents may decrease to values as low as 4 (Milinovich et al 2010). D-lactate is not produced by mammalian metabolism and therefore its presence in blood is an indicator of hindgut bacterial fermentation. In the oligofructose model, blood D-lactate concentrations peaked at approximately 20 hours after dosing, coinciding with the nadir in fecal pH (van Eps & Pollitt 2006).
Hindgut acidity triggers death and lysis of large numbers of bacteria and release of endotoxins, exotoxins and other microbial components into the intestinal milieu (Milinovich et al 2010, Pollitt & Visser 2010). There is also evidence of an increase in the synthesis of vasoactive amines within the hindgut under conditions of carbohydrate overload (Bailey et al 2003, Elliott & Bailey 2006, Crawford et al 2007). Within 24 h of carbohydrate overload, there is compromise of the intestinal mucosal barrier with degeneration and sloughing of epithelial cells, submucosal inflammation, and an increase in intestinal permeability (Sprouse et al 1987, Weiss et al 1998). The increase in intestinal permeability presumably favors absorption of exotoxins, endotoxins, other bacterial components, and vasoactive amines into the circulation. In both models, increased plasma endotoxin concentration has been detected between 8 and 12 h after starch/fructan overload (Sprouse et al 1987, Bailey et al 2009) and is associated with clinical signs of illness including tachycardia, tachypnea and pyrexia. There also is evidence of systemic inflammation with increased cytokine mRNA expression in blood, platelet activation, and activation of leukocytes (Eades et al 2007, Bailey et al 2009, Pollitt & Visser 2010). Diarrhea and clinical signs of moderate-to-severe hypovolemic shock, which may be life-threatening, are additional features of the carbohydrate overload (especially starch) models that generally occur prior to onset of lameness (Pollitt & Visser 2010).
The precise role of endotoxemia in the pathogenesis of laminitis is unclear. Intravenous infusion of endotoxin (lipopolysaccharide, LPS) does not induce development of laminitis in healthy horses (Menzies-Gow et al 2004, Tóth et al 2009). On the other hand, a clinical study has identified endotoxemia as a risk factor for laminitis in hospitalized horses although in these cases laminitis was associated with severe illness (Gram-negative sepsis) rather than nutritional factors and the diagnosis of endotoxemia was based on clinical parameters rather than direct measurement of plasma endotoxin (Parsons et al 2007). It is possible that endotoxin absorbed from the intestinal tract plays a role during the developmental phase of carbohydrate overload laminitis, perhaps by “priming” inflammatory and hemodynamic mechanisms involved in lamellar injury and failure. In support of this hypothesis, Tóth et al (2009) reported that pretreatment of horses with lipopolysaccharide (i.e., endotoxin) might increase the incidence and severity of laminitis induced by the administration of OF (5 g/kg BW). Other bacterial and non bacterial products absorbed from the gastrointestinal tract also may contribute.
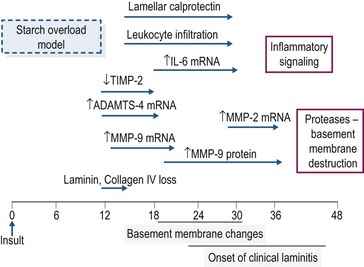
In the starch and OF overload models, clinical signs of laminitis develop 24–48 hours after bolus dosing (Pollitt & Visser 2010). Despite intensive study, the mechanism(s) that links the rapid fermentation of carbohydrate in the intestine and associated systemic responses with development of acute laminitis remains to be identified. A schematic overview of the timeline of some of the molecular and cellular events occurring during the developmental phase of starch overload laminitis is shown in Fig. 27.2. The information has been drawn from several studies in which measurements were made in laminar biopsy samples obtained at discrete time points (most often up until the onset of Obel Grade [OG] 1 laminitis; see Orsini 2010). Current evidence for the roles of inflammation, oxidant stress, matrix degradation, and venous/endothelial dysfunction is summarized below.
Lamellar leukocyte infiltration and inflammatory signaling
In both the starch and oligofructose models, there is evidence of systemic inflammation, increased expression of chemokines in lamellar tissue, plus infiltration and activation of leukocytes in lamellae (Belknap et al 2007, Falerios et al 2011a, b, Visser & Pollitt 2011a). These observations have led to the suggestion that the lamellar failure of laminitis is analogous to the organ injury and failure that occurs as a result of sepsis (Belknap et al 2007). In the starch model, there was an eightfold increase in calprotectin-positive leukocytes in laminar tissue harvested at the developmental phase (onset of fever, 10–20 hours post-dosing) with a more marked increase at the onset of OG1 lameness together with a moderate increase in CD163-positive (monocyte–macrophage) cells (Faleiros et al 2011a). Maximal leukocyte infiltration preceded development of epithelial stress and basement membrane (BM) degradation, raising the possibility that leukocyte infiltration may contribute to BM breakdown and structural failure in these models (Faleiros et al 2011b). In the oligofructose model, calprotectin-positive leukocytes also were detected in laminar tissue 18–24 hours post-dosing, although it was suggested that leukocyte infiltration and activation may be a reaction to, rather than a cause of, the lamellar pathology in this model (Visser & Pollitt 2011a, b). Increased inflammatory signaling has been detected in laminar tissue after starch or OF overload with, for example, several-fold increases in the mRNA concentrations for cytokines (interleukin (IL)-6, IL-1β), chemokines (CXCL1, CXCL8) and cell adhesion molecules (ICAM-1, E-selectin; Belknap et al 2007, Leise et al 2011). However, the majority of these inflammatory events occur at or near the onset of lameness rather than in the early developmental stages (Leise et al 2011), suggesting that other events may contribute to the initiation of lamellar pathology.
Oxidative tissue injury
Oxidant stress-associated lamellar damage may develop when there is excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), a reduced antioxidant capacity, or both (Roberts et al 2009). As equine laminar tissue is devoid of superoxide dismutase (SOD) it may be highly susceptible to damage by the superoxide anions (Loftus et al 2007). Increased lipid (increased 4-hydroxynonenal [4-HNE]) and protein (increased 3-nitrotyrosine) oxidant stress has been observed in laminar tissue after BWE administration but not in tissues harvested during the developmental and OGI phases of starch overload laminitis (Loftus et al 2007, Burns et al 2011). More work is needed to elucidate the role of oxidative stress in the pathogenesis of laminitis; however current evidence indicates that tissue oxidative stress is not central to the development of starch overload laminitis (Burns et al 2011).
Enzymatic dysregulation
Disruption of the lamellar BM is a primary lesion in carbohydrate overload laminitis. Some studies have concluded that up-regulation of matrix metalloproteases (MMPs), in particular MMP-2 and MMP-9, is responsible for the BM damage (as reviewed by Clutterbuck et al 2010) but other work has indicated that degradation of the lamellar BM occurs prior to changes in MMP (2 and 9) expression and activation (Visser & Pollitt 2012). The protease aggrecanase-1 (ADAMTS-4) is upregulated very early in the developmental phase and its role in BM degradation merits further investigation (Kyaw-Tanner et al 2008).
Alterations in vascular and endothelial function
The role of altered digital hemodynamics in laminitis is controversial with both increased and decreased perfusion detected in experimental models (see Robertson et al 2009). Nonetheless, venous dysfunction may contribute to lamellar injury in concert with other mechanisms. Laminar edema due to venous constriction has been observed during the developmental phase of carbohydrate overload laminitis (Allen et al 1990). Concurrently, there is an increase in digital venous endothelin-1 (Eades et al 2007) which has been shown to induce intense constriction of laminar veins (Keen et al 2008). The increased venous resistance during the prodromal phase can be inhibited by administration of an endothelin-1 antagonist (Eades et al 2006). Other mechanisms contributing to vasoconstriction might include localized platelet activation (with release of serotonin and thromboxane) and the formation of platelet–neutrophil aggregates, which has been demonstrated in carbohydrate overload laminitis (Weiss et al 1997). Vasoactive amines formed by bacteria in the intestinal tract, such as tyramine, tryptamine and phenylethylamine, also may contribute to altered digital hemodynamics although direct evidence is lacking (Elliott & Bailey 2006). Horses and ponies with IR and pre-existing endothelial cell dysfunction may be more susceptible to digital vasoconstriction under conditions of carbohydrate challenge and/or hyperinsulinemia (Geor & Frank 2009, Robertson et al 2009, Berhane et al 2009a,b).
Clinical relevance
The relevance of the OF model to pasture-associated laminitis is less certain. The fructans in grasses are very different in structure to the simple oligofructoses used in the experimental studies and even if similar “triggering” amounts of fructan can be ingested whilst out on pasture this occurs over several hours rather than as a bolus. Nonetheless, clinical observations have suggested that risk of laminitis is highest when horses or ponies are grazing lush (i.e., green, actively photosynthetic) or stressed (i.e., environmental conditions that restrict forage growth) pastures with high NSC content – fructans, simple sugars and/or starch (Longland & Byrd 2006). In Northern European countries, the fructan content of perennial ryegrass varies between <100 g and >400 g/kg of dry matter (DM) depending on the season and growing conditions. Myriad factors impact pasture NSC/water-soluble carbohydrate (WSC) contents but, in general, it is highest in spring, lowest in mid-summer and intermediate in the fall. In a recent study, analysis of 245 samples of pasture grasses (perennial ryegrass, timothy and fescue) harvested throughout a growing season showed that ~20% of samples contained >20% WSC (fructans + simple sugars) on a DM basis, ~5% of samples with >25% WSC, and ~3% with >30% WSC (Annette Longland, personal communication). The average fructan content of ryegrass samples harvested in May was 27% of DM. There also can be marked daily fluctuations in forage NSC that coincide with patterns of energy storage (photosynthetic activity) and utilization. During periods of growth and intense photosynthetic activity, pasture WSC tends to rise during the morning, reaching maxima in the afternoon and then declining overnight. In one study of spring pasture in northern Virginia, the nadir in forage WSC occurred between 0400 h and 0500 h (~15% WSC, DM basis), with peak values between 1600 h and 1700 h (~22–24% WSC) (Byrd et al 2006).
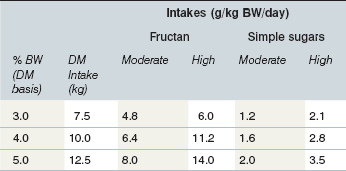
Under some conditions the intake of pasture WSC by grazing equids may approach the amount (as OF) known to induce laminitis when administered as a single dose (Longland & Byrd 2006; Table 27-2). Dry matter intake of horses with free access to pasture may exceed 3% of BW per day (i.e., 15 kg DM intake for a 500-kg horse), while ponies may consume as much as 5% of BW (Longland et al 2011a). A 250-kg pony at pasture with a WSC content of 35% DM (with 80 : 20 ratio of fructan to simple sugars) would need to consume only about 4.7 kg DM forage (1.9% of BW) to achieve a fructan dose of 5 g/kg BW, which is equivalent to the dose of OF used by Kalck et al (2009) to induce laminitis in some healthy animals. This amount of forage could be ingested in as little as 5–6 h; over a 24-h period in which total forage consumption might approach 5% of BW (12.5 kg DM) fructan intake would approach 14 g/kg BW. It is possible that the WSC dosage required to trigger digestive and metabolic disturbances in susceptible animals (i.e. animals with the EMS phenotype) is lower than that needed to reliably induce disease in healthy experimental animals. Another possibility is that “sub-threshold” doses of WSC consumed over several days induce multiple subclinical insults, with cumulative damage to the lamellae that ultimately manifests as clinical laminitis.
Insulin-induced laminitis
In healthy ponies and horses, the administration of a prolonged (~48 h) euglycemic hyperinsulinemic clamp (EHC) via simultaneous infusions of insulin and glucose induces Obel grade 1–2 laminitis (Asplin et al 2007, De Laat et al 2010). In this model, the induction of supraphysiological hyperinsulinemia (serum insulin ~1000–1100 mU/l) is accompanied by pronounced digital pulses and a significant increase in hoof wall surface temperature (De Laat et al 2010). Pathological features are similar to those described in the OF overload model, although marked leukocyte infiltration and inflammation do not appear to be features of the insulin model (Asplin et al 2010, De Laat et al 2012). The mechanism by which high circulating insulin concentrations (and perhaps also increased glucose flux) induce laminitis has not been elucidated and is the subject of current research. The involvement of increased matrix metalloproteinase activity is unlikely (De Laat et al 2011a). Insulin-like growth factor (IGF)-1 receptor has been identified on lamellar epithelial cells (Bailey & Chockalingham 2009) and it has been proposed that insulin signaling via these receptors may be involved in the pathogenesis of insulin-induced laminitis.
Clinical relevance
Findings from the insulin model have implications for nutritionally associated laminitis, providing a potential explanation for episodes of disease following consumption of feeds and forages that elicit pronounced insulinemic responses. Indeed, it has been argued that hyperinsulinemia is the unifying factor in endocrinopathic and pasture-associated laminitis (McGowan, 2010). Studies of grazing horses have shown a positive relationship between pasture NSC content and circulating insulin concentrations (Byrd et al 2006), and marked exacerbation of hyperinsulinemia has been observed in ponies with an insulin-resistant (EMS) phenotype when they are grazing spring pasture (NSC ~15–18% DM; Treiber et al 2006, 2008). In healthy, nonobese Thoroughbred mares grazing spring (April) pasture, serum insulin concentrations followed a circadian pattern that mirrored changes in forage NSC content, with peak insulin concentrations approaching 100–110 mU/l (Byrd et al 2006). Serum insulin concentrations in Welsh and Dartmoor ponies kept at pasture, some of which were insulin resistant and prone to recurrent pasture laminitis, increased markedly during the months of April and May (values as high as 600–700 mU/l). This occurrence coincided with an increase in pasture grass NSC content and the incidence of laminitis (Treiber et al 2008). Additionally, feeding inulin (to simulate intake of fructan from spring grass) to ponies can elicit an exaggerated insulin response in insulin resistant ponies that are predisposed to laminitis (Bailey et al 2007). Seasonal influences on insulin dynamics also may influence risk of pasture laminitis. A recent study demonstrated exaggerated post-dexamethasone insulin responses in previously laminitic ponies in April and July when compared to responses in December. As well, the increase in serum insulin concentrations in response to dexamethasone administration was significantly higher in previously laminitic than in normal ponies (Bailey et al 2007, Borer et al 2009). It is therefore possible that episodes of laminitis in pasture kept animals, especially those with an EMS phenotype, are directly linked to increases in circulating insulin associated with the consumption of NSC-rich forage.
Whether or not hyperinsulinemia plays an essential or exclusive role in the development of endocrinopathic laminitis remains to be determined. Although high (>750–1000 mU/l) serum insulin concentrations have been detected (Treiber et al 2008) in ponies with clinical laminitis, the authors also have observed similar high or even higher insulin concentrations in ponies without evidence of clinical laminitis. Conversely, some laminitis-prone animals do not exhibit abnormal insulin dynamics. It is possible that other factors contribute to lamellar pathology in the insulin model, for example the effects of an increase in glucose flux during the prolonged EHC. To try and address this question, healthy horses were recently subjected to a 48-h IV glucose infusion that delivered an amount of glucose equivalent to that administered in the EHC experiments (De Laat et al 2011b). This protocol resulted in marked hyperglycemia (~11–12 mmol/l), moderate endogenous hyperinsulinemia (~200 mU/l), and histological evidence of mild lamellar pathology in at least one foot of all treated horses (but not clinical laminitis). When these findings were interpreted in light of previous studies, De Laat et al concluded that: “laminitis may be induced by either insulin alone or a combination of insulin and glucose, but likely not by glucose alone”. The authors also suggested that a potential threshold for development of insulin-induced lamellar pathology may be at or below a serum concentration of ~200 mU/l (De Laat et al 2011b). However, in our view these conclusions should be interpreted with considerable caution pending further research. First, the experimental design precluded definitive conclusions regarding cause of the observed lamellar lesions. For example, a protocol that involved suppression of endogenous insulin secretion (e.g., by simultaneous infusion of somatostatin) would be needed to specifically address the hypothesis that laminitis is induced by increased glucose flux. Second, and as mentioned above, the clinical observation of profound hyperinsulinemia in animals without evidence of clinical laminitis raises several questions regarding the cause-and-effect relationship between circulating insulin and laminitis. For example, it is possible that some animals develop tolerance to the effects of high circulating insulin; if so, it will be impossible to determine a threshold of hyperinsulinemia that portends development of laminitis in all animals. It must also be recognized that recurrent laminitis can occur in some animals with no apparent background of high insulin concentrations. As well, it is possible, even likely that another mechanism(s) contributes to laminitis susceptibility in high-risk animals with an insulin resistant (EMS) phenotype – see below.
Other factors potentially increasing susceptibility to laminitis
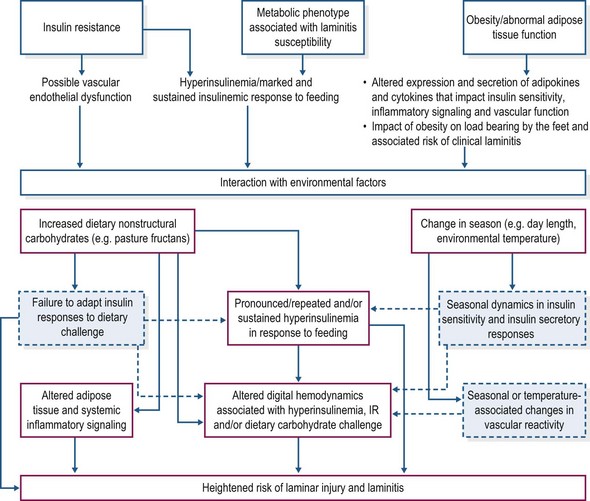
Aside from hyperinsulinemia, there may be other factors that render certain individuals more susceptible to laminitis (Fig. 27.3). Insulin resistance in humans and animal models of metabolic syndrome is characterized by vascular endothelial dysfunction that contributes to development of hypertension. Insulin is a vasoregulatory hormone, invoking vasodilatation through pathways similar to those of insulin-mediated glucose metabolism (Kim et al 2006). In insulin resistant states, insulin’s ability to counteract endothelin-1 associated vasoconstriction may be compromised due to decreased nitric oxide synthesis, while compensatory hyperinsulinemia might stimulate increased endothelin-1 production (Kim et al 2006). The resulting imbalance between the production of nitric oxide and secretion of endothelin-1 favors vasoconstriction and also contributes to platelet activation and leukocyte adhesion, all of which have been proposed as pathophysiological mechanisms in the development of carbohydrate overload-induced laminitis (Bailey et al 2004a,b, Eades et al 2007). Bailey et al (2008) documented hypertension in insulin resistant, laminitis prone ponies at summer pasture, suggesting that vascular endothelial dysfunction also is a component of the metabolic syndrome phenotype in equids. EMS animals may therefore be more susceptible to digital vasoconstriction, platelet aggregation, and neutrophil adherence and emigration into lamellar tissues under conditions of alimentary carbohydrate overload (Geor & Frank 2009).