Chapter 60 Lactic Acidosis
LACTATE METABOLISM
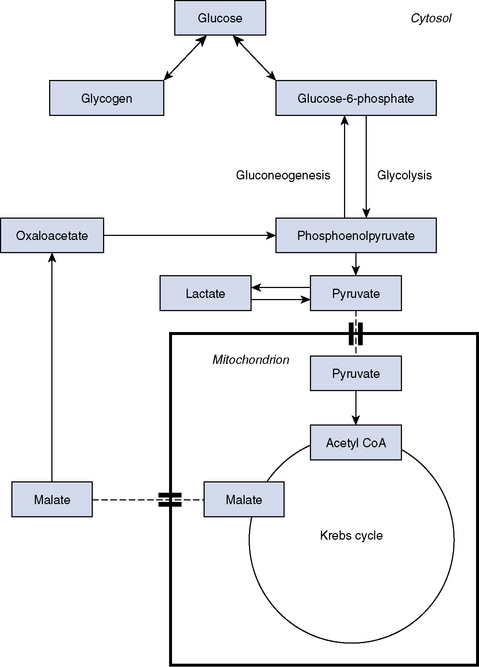
Lactate as an organic acid is unremarkable and behaves as do many other intermediary metabolites. What makes it remarkable is that it exists in a metabolic cul-de-sac with no way in or out except through pyruvate. During anaerobic metabolism when pyruvate is not efficiently metabolized via the Krebs cycle, lactate is formed in high concentration. This can occur with exercise or with disease. Lactate synthesis serves to regenerate nicotinamide adenine dinucleotide, an essential reducing equivalent for ongoing glycolysis. When aerobic conditions are restored, pyruvate is cleared, which opens the pathway for lactate to be metabolized back to normal levels. Lactate may also be transported in the blood and used in the liver under aerobic conditions for energy storage via gluconeogenesis and glycogen synthesis. This pathway involves reconversion of lactate to pyruvate in the liver (Cori cycle) under aerobic conditions to drive the Krebs cycle (Figure 60-1).4,6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


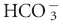 ].1-3 In the conventional approach to acid-base analysis, lactic acidosis is considered to be the consequence of the hydrogen ion production that occurs in conjunction with lactate metabolism. When the lactate anion is in association with another cation such as sodium, as it is in lactated Ringers solution, it does not have an acidifying effect.1,3,4
].1-3 In the conventional approach to acid-base analysis, lactic acidosis is considered to be the consequence of the hydrogen ion production that occurs in conjunction with lactate metabolism. When the lactate anion is in association with another cation such as sodium, as it is in lactated Ringers solution, it does not have an acidifying effect.1,3,4