Chapter 196 Aminoglycosides
PHARMACOLOGY AND DOSING
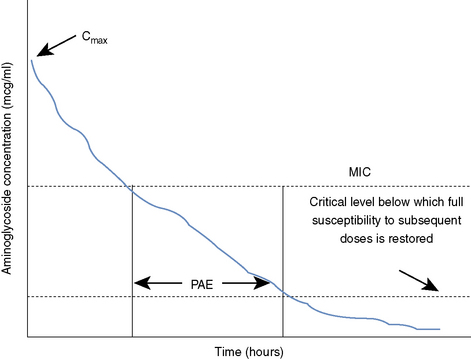
Aminoglycosides provide a PAE, which means that bacterial replication is impeded even after serum drug concentrations have fallen below the minimum inhibitory concentration (MIC) of the organism in question (Figure 196-1).1,3,4,6 This permits longer dosing intervals. The postantibiotic effect (PAE) tends to be longer in vivo than in vitro.1 Aminoglycosides possess a PAE that is linked to (1) the species of bacteria, (2) the MIC of the bacterial strain, and (3) the concentration of drug achieved at the site of infection.6 The PAE demonstrated by aminoglycosides is one component of SDD that allows extended drug-free intervals without compromising patient outcome, and it may be enhanced by higher dosages and concurrent administration of a cell wall–active antibiotic such as a β-lactam.13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree