Chapter 122 Blood Film Evaluation
• Blood film evaluation provides both general information regarding the health status of emergent and critically ill patients and specific diagnostic information in the case of true hematologic emergencies. It is therefore an essential component of the workup for all emergency and critical care patients.
• Blood film evaluation requires a systematic approach. The various cellular elements of the blood (leukocytes, erythrocytes, platelets) should be examined in the same order and manner on every blood film.
• Leukocyte morphology is used to recognize and characterize inflammatory disease. Signposts of inflammation are a neutrophilic left shift, monocytosis, or a persistent eosinophilia. Regenerative left shifts (high neutrophils, increased bands) indicate that bone marrow response is keeping pace with tissue demand; degenerative left shifts (low or normal neutrophils, increased bands) indicate that tissue demand is overwhelming the bone marrow (guarded prognosis).
• Neutrophil toxicity indicates either the presence of circulating toxins interfering with neutrophil development in bone marrow or accelerated neutrophil production. Neutrophil toxicity is most commonly (but not exclusively) associated with bacterial infection.
• Lymphocyte patterns and morphology are also important in critically ill patients. Low lymphocyte numbers are generally a reflection of stress (high circulating corticosteroids). Reactive lymphocytes indicate antigenic stimulation.
• The critical step in evaluating anemias is classifying them as regenerative or nonregenerative based upon the presence (regenerative) or absence (nonregenerative) of increased numbers of polychromatophils on the blood film. Regenerative anemias are due to blood loss or hemolysis, whereas nonregenerative anemias have a broader range of causes.
• In highly regenerative anemias, red blood cell morphology is extremely important, because it may indicate a specific hemolytic disease. Immune-mediated hemolytic anemia (IMHA), Heinz body hemolytic anemia, mycoplasmosis, and babesiosis, among others, are all hemolytic diseases that can be diagnosed largely on the basis of red blood cell morphology.
• Thrombocytopenia is the most common primary cause of bleeding disorders in dogs and cats. Thrombocytopenia can result from sequestration of platelets in an enlarged spleen (rare), peripheral utilization in severe inflammation (clinical or subclinical disseminated intravascular coagulation [DIC]), immune-mediated peripheral destruction (immune-mediated thrombocytopenia [ITP]), or lack of production in the bone marrow.
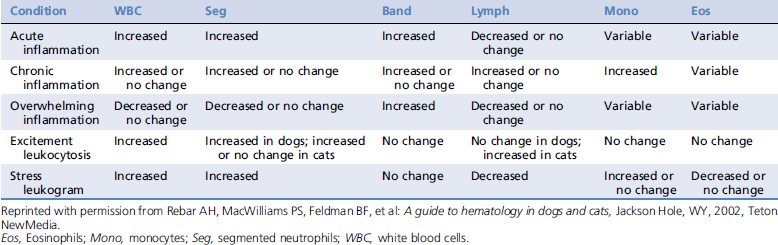
WHITE BLOOD CELL RESPONSES1,3
White blood cell responses are among the best laboratory the indicators of the general health status of critically ill patients. Many emergent and critically ill patients suffer from inflammatory diseases. Inflammation is usually indicated by the leukogram. Signs of inflammation include a left shift, monocytosis, or persistent eosinophilia. General patterns of leukocyte responses are found in Table 122-1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree