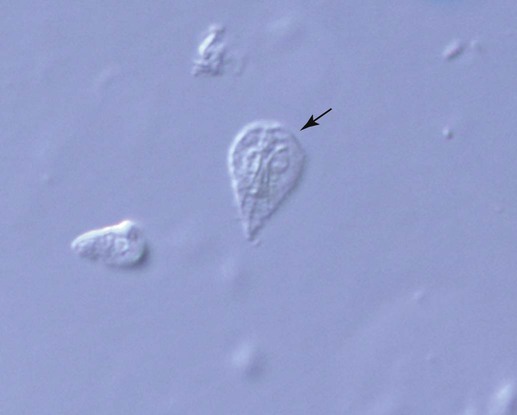
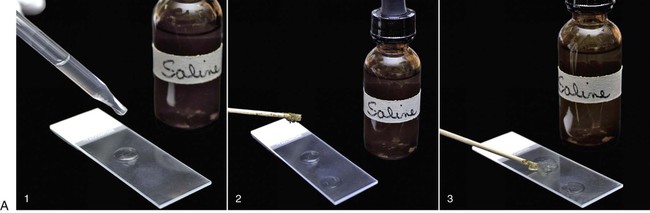
To maximize the likelihood of identifying a parasite, at least 5 g of feces should be collected and placed in a clean, dry, air-tight container. The amount of material retrieved by use of a fecal wand is insufficient for reaching a diagnosis of most intestinal parasites. Although interpretation of a positive result on direct smear from a fecal wand sample is possible, a negative result is all but meaningless because of the small sample size examined. If possible, the fecal sample should be held at room temperature and examined immediately (within 30 minutes) after collection. Protozoal trophozoites may lyse during refrigeration or long-term storage and thus fail to be detected. If samples cannot be examined immediately, however, feces should be refrigerated. Tightly sealed, refrigerated samples may be examined for the presence of cysts or oocysts, which are hardier and persist in the environment for up to 1 week after collection.7 When Tritrichomonas foetus is suspected, some clinicians prefer the saline flush technique to collect a fecal sample; specimens obtained from nondiarrheic or dry stools are unsuitable for use in testing for T. foetus. Stopping antibacterials or antiprotozoals for several days before testing for T. foetus is also recommended, because use of these drugs may interfere with the detection of T. foetus.14 The saline flush technique consists of passing a rubber catheter into the proximal colon followed by instillation and recovery of 10 mL of sterile saline. Water should not be used, because this will lyse the trophozoites. The sterile saline is injected through the catheter into the colon and then gently reaspirated. The fecal-saline solution is then examined directly under the microscope for trophozoites, or a drop is inoculated in to a fecal culture pouch for parasite isolation (see Tritrichomoniasis, Chapter 77). Alternatively, the saline flush contents are sedimented by centrifugation and the pellet submitted for polymerase chain reaction (PCR) analysis.14 Table 70-1 lists the recommended microscopic procedures for the diagnosis of intestinal protozoal infections. Because dogs and cats are predators and dogs are coprophagous, some protozoal stages found in fecal samples may be something the animal has ingested rather than a result of true parasitism. For example, oocysts of Eimeria spp. are often observed in canine fecal samples because the dog has ingested herbivore feces, usually those of a rabbit, deer, horse, or cow. True parasitism is not present in the dog in these cases, and treatment is not indicated. TABLE 70-1 The direct smear technique, which can be performed on diarrheic or formed fecal samples, is most important when used with diarrheic specimens. To enhance detection, the substage condenser of the microscope should be adjusted to maximize contrast; unstained organisms may otherwise be overlooked. When available, the phase-contrast or dark-field microscope may aid in the demonstration of motile trophozoites (Fig. 70-1), but these are not necessary for reliable diagnosis of protozoal infection. A direct smear is made by placing a drop of isotonic saline on a clean microscope slide and then mixing in a minute amount of fecal material (Fig. 70-2, A); the amount that adheres to the tip of a wooden applicator stick is sufficient. A coverslip is then applied, and the slide systematically scanned using the 10× objective but alternating with the 40× objective whenever structures morphologically resembling protozoal trophozoites or cysts are encountered. The fecal-saline mixture should be transparent enough that newsprint can be easily read through the material; a smear prepared with too much material cannot be accurately interpreted. Motility and structural features of protozoa are best examined using the high-dry objective (40× to 43×). The oil immersion objective (100×) is not recommended for examining fresh wet mounts because pressure on the coverslip from the oil objective displaces the fluid medium between the coverslip and the slide, making viewing difficult. If necessary, once motility has been observed, stain may be added to the smear to aid in specific identification of the organisms.16 Although not required, adding stain to the wet mount through the edge of the coverslip may aid in visualizing internal structures of some protozoa. Because staining the preparation kills the organism, examination for motility must be performed first. Table 70-2 describes the trophozoites found on direct smears from fecal samples of dogs and cats. Stain can be very useful for identifying certain protozoa. For example, although Pentatrichomonas hominis usually has five anterior flagella and T. foetus usually has three anterior flagella, it is extremely difficult to identify them using living or unstained specimens. Special stains such as silver staining are needed to highlight flagella and other diagnostic features of the trophozoites so morphologic diagnosis can be accomplished.11 TABLE 70-2 Protozoal Trophozoite Identification by Direct Fecal Smear Iodine is the most common stain used to reveal the internal structure of protozoans and is especially useful for confirming the identity of Giardia cysts and trophozoites. The iodine stains the cytoplasm of cysts dark gold, while the nuclei remain pale and refractile. Other stains used to identify protozoa in feces include methylene blue, acid methyl green, aqueous eosin, and crystal violet. Methylene blue is useful for identifying trophozoites, especially those of Entamoeba histolytica, whereas acid methyl green stains the macronucleus of Balantidium coli. Aqueous eosin and crystal violet are both negative stains, which stain fecal debris but leave parasitic cysts and trophozoites, which exclude the dye, highlighted by their colorless appearance against the stained background.2 Solutions used in centrifugal fecal flotation methods include zinc sulfate and Sheather’s sugar. Five to 10 g of feces is mixed with water to a liquid consistency, and the mixture is strained with gauze. Two parts Sheather’s sugar solution (500 g sugar, 300 mL water, and 6.5 g melted phenol crystals) are added to one part fecal suspension in a capped centrifuge tube. Care should be taken not to fill the tube to the top to prevent spills or aerosols. After centrifugation at 1000 × g for 10 minutes, one or two drops from the meniscus are removed by a dropper, placed on a microscope slide, covered with a coverslip, and examined at low power (×100) magnification. To assist in the examination of very mucoid or fatty fecal samples, feces should first be washed by combining 2 to 3 g of feces with 5 to 10 mL of water, straining the mixture through a double layer of cheesecloth, centrifuging for 5 to 10 minutes at approximately 650 × g, and then discarding the supernatant. The sediment should then be mixed with 5 to 10 mL 33% zinc sulfate solution with a specific gravity of 1.18 or Sheather’s sugar solution, strained through cheesecloth, and centrifuged for 5 to 10 minutes at approximately 650 × G.16 When available, a centrifuge with a swinging bucket rotor is ideal for the flotation step (Fig. 70-2, B). With this protocol, the strained feces-flotation solution mixture is poured into a 15-mL conical centrifuge tube, and enough flotation solution is added to form a reverse meniscus at the top of the tube. A coverslip is then placed on top of the meniscus and the tube spun with the coverslip in place; a second tube of equal volume is needed to balance the centrifuge. Once completed, the coverslip is removed and transferred along with the adherent fluid face down on a slide, and the slide is examined (Fig. 70-2, B). If only a fixed-angle bucket rotor is available, fecal flotation with centrifugation can still be performed. In this case, the feces-flotation solution mixture should be poured into a 15-mL centrifuge tube to the point at which it is almost full but does not spill when placed into the centrifuge bucket (approximately 13 mL). The tube is then spun without a coverslip. After centrifugation is complete, the top layer of the centrifuged material can be collected by carefully touching the base of a small test tube or a microbiologic loop to the surface and then transferring the adherent fluid to a slide. A coverslip is then applied and the preparation examined. Prepared slides should be completely examined using the 10× and, when necessary, the 40× objective to confirm the identification of an organism. Scanning a slide at low power (×4) is not sufficient to allow detection of most protozoa.16
Laboratory Diagnosis of Protozoal Infections
Microscopic Examination
Feces
Collection and Storage of Feces
Examination of Feces
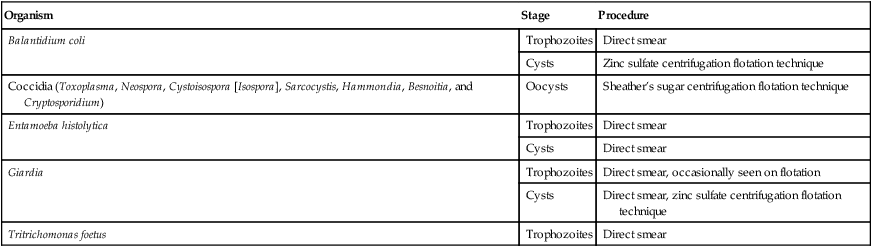
Organism
Stage
Procedure
Balantidium coli
Trophozoites
Direct smear
Cysts
Zinc sulfate centrifugation flotation technique
Coccidia (Toxoplasma, Neospora, Cystoisospora [Isospora], Sarcocystis, Hammondia, Besnoitia, and Cryptosporidium)
Oocysts
Sheather’s sugar centrifugation flotation technique
Entamoeba histolytica
Trophozoites
Direct smear
Cysts
Direct smear
Giardia
Trophozoites
Direct smear, occasionally seen on flotation
Cysts
Direct smear, zinc sulfate centrifugation flotation technique
Tritrichomonas foetus
Trophozoites
Direct smear
Direct Saline Smear
Stained Smear
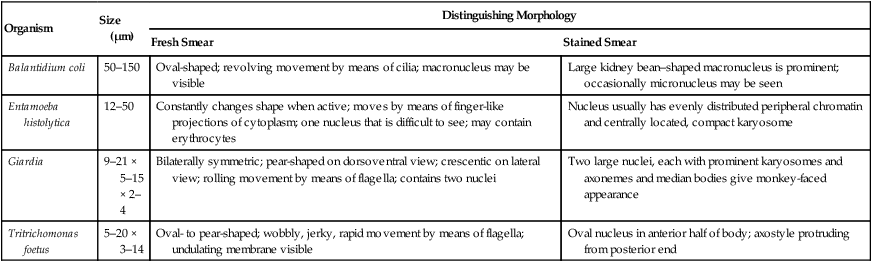
Organism
Size (µm)
Distinguishing Morphology
Fresh Smear
Stained Smear
Balantidium coli
50–150
Oval-shaped; revolving movement by means of cilia; macronucleus may be visible
Large kidney bean–shaped macronucleus is prominent; occasionally micronucleus may be seen
Entamoeba histolytica
12–50
Constantly changes shape when active; moves by means of finger-like projections of cytoplasm; one nucleus that is difficult to see; may contain erythrocytes
Nucleus usually has evenly distributed peripheral chromatin and centrally located, compact karyosome
Giardia
9–21 × 5–15 × 2–4
Bilaterally symmetric; pear-shaped on dorsoventral view; crescentic on lateral view; rolling movement by means of flagella; contains two nuclei
Two large nuclei, each with prominent karyosomes and axonemes and median bodies give monkey-faced appearance
Tritrichomonas foetus
5–20 × 3–14
Oval- to pear-shaped; wobbly, jerky, rapid movement by means of flagella; undulating membrane visible
Oval nucleus in anterior half of body; axostyle protruding from posterior end
Fecal Flotation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Laboratory Diagnosis of Protozoal Infections