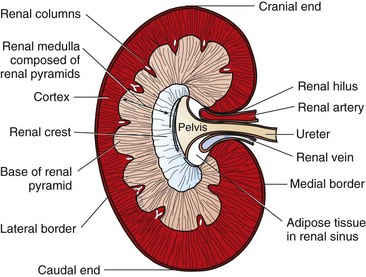
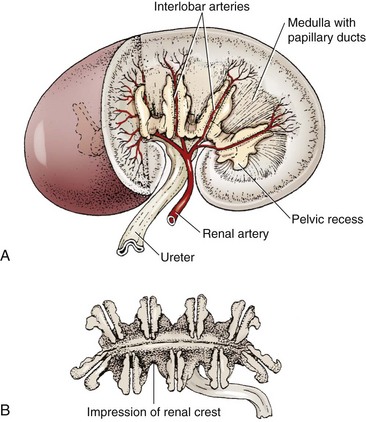
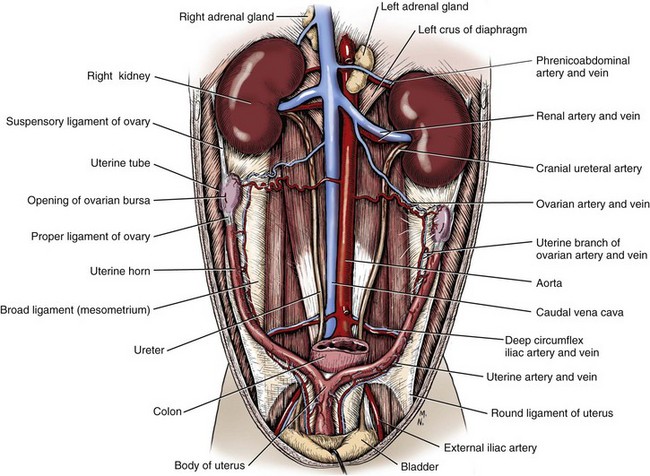
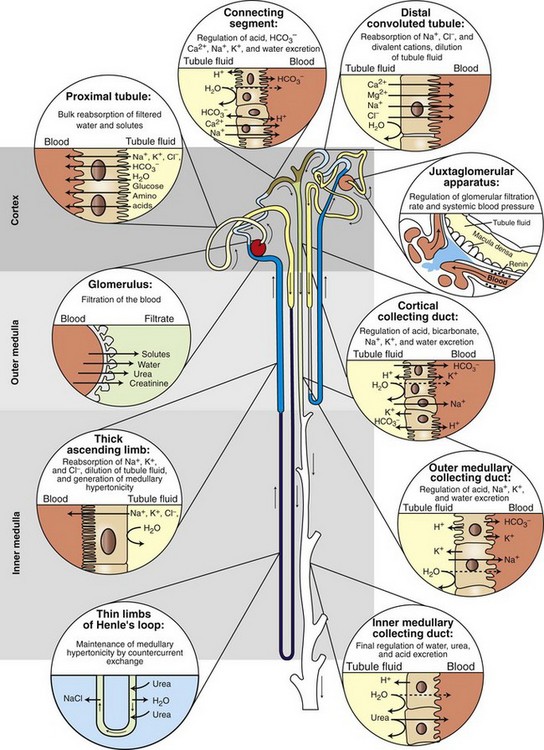
Chapter 114 Each kidney has a cranial and caudal pole and a ventral and dorsal aspect (Figure 114-1). The concave surface of the kidney is located along the medial aspect and is called the hilus. The hilus is the location where the renal artery enters the kidney and the renal vein and ureter exit. Nerves and lymphatic vessels enter at the hilus as well. Anatomically, the renal vein is located more ventrally, and the renal artery is more dorsally. In an animal of normal body condition, the kidney is typically surrounded by a substantial amount of fat; this fat is maintained even in lean animals. In obese animals, the surrounding adipose tissue can virtually hide the kidney from view, making gross evaluation difficult. On gross dissection, the kidney is divided into several distinct areas. Outermost, there is a thin, fibrous capsule covering the kidney. In a healthy kidney, the capsule is easily separated from the underlying parenchyma. In a diseased kidney, the capsule may be thickened and tightly adhered to the underlying renal tissue. On cross-section, the kidney is divided into the outer cortex and the inner medulla. The renal cortex is composed of glomeruli and adjacent structures. The medulla is striated in appearance and projects into the renal pelvis as the renal crest (Figure 114-2). The renal pelvis is the space into which urine is deposited by the renal collecting ducts and is continuous with the ureter. The renal arteries arise directly off of the abdominal aorta (Figure 114-3). Although most kidneys are supplied by a single renal artery, multiple renal arteries are reportedly seen in 13% of canine kidneys and in 10% of feline kidneys.23 The left kidney is more likely to have multiple renal arteries than the right kidney. Some single renal arteries branch immediately after leaving the aorta, making it appear that the kidney has two renal arteries. This is primarily of significance during nephrectomy, when severe hemorrhage can be encountered if one of these renal artery branches is not identified and ligated before transection. Small “capsular” arteries may enter the kidney from the capsular surface. These secondary arteries anastomose to the primary renal arteries and create a renal “arterial circle” that permits some arterial flow to a kidney even when a renal artery is obstructed. Capsular arteries most commonly arise from the phrenicoabdominal artery and adrenal arteries23 and appear to be more prominent in diseased kidneys. The vasa recta is composed of long, unbranched capillaries that extend alongside the nephron from the cortex into the renal medulla. Through these capillaries, water reabsorbed from the collecting tubules is returned back into the circulatory system, helping to maintain hypertonicity of the renal medulla through a countercurrent exchange system.14,15,23,39 The venous circulation of the kidney arises from deep and superficial veins within the renal parenchyma. Veins in the outer cortex drain toward the capsular surface. These veins converge to form the stellate veins, interlobar veins, arcuate veins, and renal vein. The renal vein returns blood back into the caudal vena cava. The left renal vein also receives blood from the left ovarian or testicular veins. Capsular and parenchymal lymphatics converge to make lymphatic trunks, which exit at the renal hilus.23,27 The basic functional unit of the kidney is the nephron (Figure 114-4). Dogs have an estimated 500,000 nephrons per kidney. The nephron consists of the renal corpuscle and the renal tubules, which end at the collecting duct. The renal corpuscle, which is found only in the renal cortex, is composed of the glomerulus and the glomerular capsule (also referred to as Bowman’s capsule). The glomerulus is a tuft of capillaries enclosed within the glomerular capsule. Between the glomerulus and the afferent arterioles is a group of cells called the macula densa. These cells help maintain autoregulation of renal blood flow. The glomerular capsule is the beginning of the tubular system that is responsible for the formation and transport of urine. It is composed of highly specialized epithelial cells called podocytes, which rest on the glomerular basement membrane. Interdigitation of podocyte appendages creates filtration slits, or pores, between the cells. The size of these filtration slits is the primary determinant of which particles can filter out of the blood and into the renal collecting tubules. Water and small particles (<60,000 daltons) can freely pass through these filtration slits, but particles larger than 60,000 daltons have very limited ability to pass through. Most cellular components of blood exceed this size; thus, the presence of these components in the urine may be an indication of significant glomerular damage. An inherent negative charge to the glomerular basement membrane may also help to repel other negatively charged molecules, such as albumin, enhancing the selective nature of glomerular filtration.92 Injury to the glomeruli (glomerulitis) can result in a loss of podocytes, resulting in an inefficient and indiscriminate filtration mechanism. Glomerular disease may result in edema formation, systemic hypertension, thromboembolism, hyperlipidemia, protein malnutrition, immunosuppression, changed pharmacokinetics of excreted drugs, and endocrine abnormalities.92 Normal urine production in dogs and cats varies between 20 and 45 mL/kg/d.2 Urine begins as a filtrate that is created as water and small particles move through the filtration slits. Glomerular filtrate is roughly 300 mOs/L (roughly equal to plasma). The filtrate then drains into the tubular system that begins with the proximal convoluted tubule (see Figure 114-4). From this point, the filtrate moves into the descending loop of Henle as it crosses from the renal cortex into the medullary region. The loop of Henle makes a hairpin turn in the medulla, becoming the ascending loop of Henle. The tubule system crosses again into the cortical region, becoming the distal convoluted tubule. Filtrate in the distal convoluted tubule drains into the collecting tubule and moves into the papillary duct and the collecting system. Creation of dilute urine requires the resorption of solutes from filtrate, while the formation of concentrated urine requires the resorption of water from the filtrate. These processes are accomplished in the various portions of the nephron. The concentrating ability of kidneys varies among species, depending on the environmental requirements of the animal. For instance, animals in aquatic environments have limited concentrating ability (beavers, 500 mOs/L), but desert creatures have the ability to produce extremely concentrated urine (desert mice, 10,000 mOs/L).39 Healthy humans can produce urine between 1200 and 1400 mOs/L. When vascular injury is minimal, connective tissue and collagen rapidly repair renal parenchymal wounds. Inflammation and infarction occur with parenchymal ischemia secondary to compression, electrocoagulation, vascular transection, or inflammation and delay wound healing.8 Intraparenchymal hemorrhage increases the amount of fibrosis and renal damage, resulting in obliteration of local nephrons and dilatation and obstruction of renal tubules in the area of the hematoma.34,86 Use of transparenchymal horizontal mattress sutures to stem hemorrhage, however, causes parenchymal necrosis, fibrosis, scarring, and atrophy.34 Healing is more rapid when intraparenchymal vessels are individually ligated. In general, a complete blood count, biochemistry and coagulation panels, urinalysis, urine culture, and blood pressure should be evaluated in any animal undergoing renal surgery.72,90 Systolic blood pressure greater than 180 mm Hg is considered abnormal.94 The risk of hemorrhage during or after surgery is increased in patients with azotemia, hypertension, or thrombocytopenia.10 Buccal mucosal bleeding time is recommended in animals with uremia, which impairs platelet adhesion and aggregation.10 A cross-match should be performed in any animal with coagulopathy or anemia or in which excessive bleeding is expected. In one study, coagulation panels were abnormal in 39.8% of dogs and 51.9% of cats tested before renal biopsy.94 Thoracic radiographs should be performed in any animal in which neoplasia is suspected. At the time of diagnosis, pulmonary metastases are detected on thoracic radiographs in 16% to 48% of dogs with primary renal tumors; 77% have metastatic disease at the time of death.17,49 In cats with nonlymphomatous primary renal neoplasia, pulmonary metastases are diagnosed preoperatively in 43%.42 Thoracic radiographs are also recommended in patients that are dyspneic or have undergone trauma. Hydrothorax and urothorax have been reported in animals with perirenal cysts and diaphragmatic renal herniation, respectively.77,87 Abdominal radiographs and renal ultrasonography are performed in most affected animals to evaluate renal structure, identify and locate calculi, examine the kidneys and other organs for primary or metastatic neoplasia, and obtain fluid or tissue samples. Renal function of the contralateral kidney should be evaluated before unilateral nephroureterectomy.52 The kidneys are easily identified on routine survey films of the abdominal cavity. Their fixed retroperitoneal position provides a consistent location, and the large amount of perirenal fat provides differential contrast. A right lateral projection is preferred because this view maximizes separation of the kidneys, resulting in a better lateral image of each.28 Radiographically, a normal canine kidney is 2.0 to 2.5 times the length of the adjacent vertebra, and a normal feline kidney length is 2 to 3 times the adjacent vertebrae. Radiographic imaging of the kidney can be enhanced by administration of intravenous (IV) contrast agents that are filtered or excreted by the urinary system. The resulting study is called an excretory urogram or intravenous pyelogram. An intravenous pyelogram provides a qualitative assessment of each kidney and is useful in defining anatomic structures. It does not, however, provide quantitative information about renal function; contrast is removed by passive glomerular filtration and is therefore a function, but not a measurement, of glomerular filtration rate.28 Renal excretion of contrast agents should not be confused with renal function. It is entirely possible for a kidney with very little functional capacity to opacify during a contrast study. Kidneys with an abnormally low glomerular filtration rate may not be adequately visualized during a contrast study. Toxicity.: Excretory urography can be used in patients with normal renal function and in patients with evidence of preexisting azotemia as long as the animal is adequately hydrated. Because of decreased filtration, some patients with decreased renal function may require a higher dose of contrast to obtain a diagnostic study. Iodinated contrast agents have been recognized as having a certain degree of renal toxicity, making their use in some patients questionable. Development of acute renal failure after excretory urography has been attributed to contrast agents in dogs, cats, and humans.28 The exact mechanism of renal toxicity is not well understood. Systemic administration of contrast agents may result in hypotension or, when iodinated agents are used, in allergic reactions and anaphylactic shock.29 Patient Preparation.: Pre-study preparation is important to maximize image interpretation. Normally, food is withheld for 12 to 24 hours before imaging, and enemas are administered to clear fecal material from the colon. When the time available for preparation does not permit these steps, the quality of the study and its subsequent interpretation can be lessened. Patients with impaired renal function are placed on fluids before and after the contrast study to maintain hydration and encourage contrast excretion. Study Timing.: Radiographs are taken immediately after injection of the contrast agent and at 5, 20, and 40 minutes after injection. Additional films may be required, depending on how contrast excretion is progressing. In animals with renal disease in which renal contrast excretion is slow, prolonging the time between radiographs or adding additional time points may be appropriate to gain maximal information.28 The right lateral view is preferred because it increases the distance between the kidneys.28 Contrast Dose.: Contrast agents are administered systemically as a bolus injection, with the delivered volume based on iodine content (Table 114-1). The volume is calculated based on a dose of 400 mg of iodine/kg of body weight.28 This volume is considered to be adequate for most contrast studies. Table • 114-1 *Other contrast agents (or brands) may be available in different geographic locations. †Contrast agents have been associated with anaphylactic reactions, hypotension, and acute renal shutdown. Use in patients with renal insufficiency is cautioned. ‡Standard dosage is 400 mg of iodine/kg of body weight given intravenously. Phases of Contrast Excretion.: Iodinated contrast agents are normally injected intravenously and are carried by the circulation to the kidney, where the contrast is filtered by the glomeruli. An excretory urogram is split into phases that highlight various portions of the kidney at specific times. The first portion is the renal angiographic phase, which occurs almost immediately after contrast injection. This phase demonstrates the arterial supply of the kidney. The best renal angiographic studies are produced by injection of contrast into the aorta just cranial to the renal arteries, which delivers a large bolus directly to the vessels of interest. Aortic catheterization is a complex procedure, however, and is rarely performed. Although IV administration of contrast is most common for renal contrast studies, direct injection into the renal pelvis has been used.80 This procedure is indicated when there is concern about giving a systemic dose of contrast or when the renal artery is obstructed. Ultrasonography provides an excellent means for noninvasively evaluating renal structure in animals. One study of ultrasonographic evaluation of renal parenchymal disease in dogs reported that ultrasonography was most specific for focal, multifocal, and diffuse diseases of the kidney and least effective for parenchymal diseases that did not have specific-architectural renal disruption.96 Major ultrasound findings in affected dogs included renal pelvic dilatation (usually with proximal ureteral dilatation) and hyperechoic mucosal margins within the renal pelvis, proximal ureter, or both. In another study, the diagnosis of experimentally induced pyelonephritis in dogs was accurate based on results of excretory urography in 72% of dogs and on ultrasonography in 82%.67 Ultrasound guidance is routinely used to facilitate collection of renal aspirates and biopsies.38 Complications associated with ultrasound-guided kidney biopsy procedures are discussed in a later section. Resistance Index.: Doppler ultrasonic imaging can be used to determine the resistance index of the kidney. RI is defined as [(Peak systolic shift − Minimum diastolic shift)/Peak systolic shift]. Normal dogs are estimated to have an RI of 0.63 ± 0.05. By taking the mean value and adding the standard deviation, a value of 0.73 was established as the high end of normal for awake dogs. Compared with awake dogs, sedated dogs were found to have a lower mRI (0.45 ± 0.06). There was no difference between the right and left kidneys.78 RI values appear to be related to age, with younger and older individuals having higher RI values. Increased RI values have been reported in nonsedated dogs with obstructive uropathies and acute tubular necrosis. Investigators did not find a significant increase in RI in dogs with induced renal failure from aminoglycoside toxicity.79 Computed tomography (CT) provides good images of the kidney, particularly when contrast enhancement is performed. In one study comparing ultrasonography, CT, and scintigraphy in cats with normal and polycystic kidneys, noncontrast CT studies provided minimal benefit regarding cyst identification. When contrast CT was performed, however, cysts within renal parenchyma were more easily identified.76 CT angiography, using a variety of protocols, can accurately predict renal vasculature anatomy and is thought to be superior to excretory urography for evaluation of renal vasculature in potential kidney donors.12,18 In one study, CT angiography with 1-mm slices had a 92% accuracy in identifying multiple renal arteries or veins in cats.18 In that study, the most common vascular pattern was a single vessel that bifurcated before reaching the renal hilus. Cats with multiple vessels were more likely to have multiple renal veins draining the right kidney and multiple arteries supplying the left kidney.18 In another study, feline renal vascular anatomy was delineated using CT angiography and three-dimensional image reconstruction.12 Contrast-enhanced magnetic resonance angiography (MRA) has been used for quantitative and qualitative evaluation of renal vessels in humans. In a recent experimental study, MRA permitted distinguishing of renal arteries and veins in normal dogs.21 Potential applications for renal MRA include evaluation of renal vasculature in potential kidney donors or animals with renal neoplasia or vascular anomalies.16 Dynamic renal scintigraphy is used to determine the glomerular filtration rate of the kidney. Scintigraphy can be used to measure total glomerular filtration rate (total function of both kidneys) or function of an individual kidney (single-kidney glomerular filtration rate). Knowledge of single-kidney glomerular filtration rate is useful for determining the importance of that kidney to the overall renal function of the patient. Measurement of renal glomerular filtration rate is recommended as a preliminary diagnostic test whenever renal surgery, especially a unilateral total nephrectomy, is being considered. Dynamic renal scintigraphy is less accurate for evaluating glomerular filtration rate than plasma clearance studies because of its shorter sampling times. Despite the loss of some accuracy, however, scintigraphy is preferred because of the technique’s ease and speed. For most patients, renal scanning only requires placement of an intravenous catheter for administration of the radiopharmaceutical. The time for the procedure is generally less than 10 minutes (~2 minutes for the actual image acquisition), and results can be generated immediately at the end of the scan.25 Some animals will lie still on the gamma camera during the scan, but others require mild sedation. In a study evaluating the effect of various sedative combinations in dogs, the use of butorphanol and acepromazine, butorphanol and diazepam, or butorphanol and ketamine did not significantly alter the GFR when compared with results of scans performed on awake dogs.68 Variations in Glomerular Filtration Rate.: Inherent renal autoregulatory mechanisms can result in substantial variation in glomerular filtration rate of normal animals. Thus, while all values are expected to be within the normal reference range, there can be intrapatient variation with different studies because of the normal reserve capacity of the kidney. In patients with decreased renal function (e.g., kidneys with little or no remaining reserve capacity), glomerular filtration rate results are more consistent.25 Dynamic renal scintigraphy provides information on acute changes in renal glomerular filtration rate; therefore, the clinician must interpret the scan results with an understanding of the patient’s hydration status because a decrease in the glomerular filtration rate may be masked by patient diuresis.25 To avoid this confounding factor, the patient should be scanned when its hydration status is normal or near normal. Radiopharmaceuticals.: Various radiopharmaceuticals (e.g., 99mTc-DTPA [diethylenetriaminepentaacetic acid], 99mTc-MAG3 [mercaptoacetyltriglycine]) can be used to perform renal scintigraphic scans, with different compounds better suited for different situations.4,25,37,54 99mTc-DTPA is used to quantify renal function, determine total glomerular filtration rate, and quantify subclinical renal disease by allowing earlier evaluation before biochemical evidence of renal dysfunction.25 99mTc-DTPA is removed from the blood by glomerular filtration; there is no tubular secretion or tubular absorption of the compound, and it does not bind to plasma protein.25 Although the percentage uptake of 99mTc-DTPA using a gamma camera is less accurate than measurement of 99mTc-DTPA clearance from plasma, the increased sampling time, requirement for multiple blood samples, and bench-top time required to determine the clearance values make measurement of clearance less useful for clinical work. Furthermore, 99mTc-DTPA plasma clearance does not provide information about the contribution of each individual kidney to total glomerular filtration rate of the patient.4,5 This information is of vital importance when considering whether nephrectomy, nephrotomy, or partial nephrotomy is appropriate for a patient. The radiopharmaceutical compound 99mTc-MAG3 can also be used for dynamic renal scintography scans. This compound is primarily secreted by renal tubules (90%), with roughly 10% removed by glomerular filtration. 99mTc-MAG3 is a better option for determining glomerular filtration rate in patients with very limited renal function and for performing renal perfusion studies to evaluate renal transplant patients.25 99mTc-DTPA and 99mTc-MAG3 both provide single-kidney and total glomerular filtration rates of individuals. Because the mechanisms of renal excretion are different for 99mTc-DTPA and 99mTc-MAG3, surgeons need to be aware of which compound is being used when looking at the numbers generated from these scans (Table 114-2). Administration of the diuretic furosemide does not significantly change the glomerular filtration rate in normal cats and dogs undergoing 99mTc-DTPA but does decrease the half-life and speed clearance of the compound41 so that surgery can be undertaken sooner. Table • 114-2 Glomerular Filtration Rate Reference Ranges Using 99mTc-DTPA25 When possible, uremia, blood pressure irregularities, anemia, coagulopathies, and electrolyte imbalances should be corrected before anesthesia.72,90 Patients with hypoproteinemia require oncotic support (e.g., hetastarch). An indwelling urinary catheter is placed to monitor urine production before and after surgery. Patients with erythrocytosis secondary to renal neoplasia may require phlebotomy (removal of 10 to 20 mL/kg of blood) and intravenous fluids to normalize hematocrits and decrease the risk of intraoperative hemorrhage and postoperative thromboembolism. Because complication rates are high after renal surgery, medical management of nephric or ureteral calculi is usually attempted before considering surgery. Surgery is recommended for animals with complete obstruction, worsening azotemia, or unresponsive pyelonephritis.63 Maintenance of renal perfusion under anesthesia is critical; therefore, pre- and intraoperative hypotension should be corrected or prevented.32,90 Drugs that cause hypotension (e.g., acepromazine) or nephrotoxicity (e.g., aminoglycosides or nonsteroidal antiinflammatory drugs [NSAIDs]) should be avoided.31 Animals with renal dysfunction are often premedicated with an anticholinergic drug and opioids and induced with IV propofol or an inhalant anesthetic delivered by mask. Anesthesia is maintained with isoflurane or sevoflurane, and blood pressure and urine output should be monitored during the procedure. Dopamine or dobutamine may be required intraoperatively in hypotensive animals that do not respond to fluids or oncotic support. Epidural administration can reduce intraoperative anesthetic requirements and provide preemptive and postoperative analgesia. Renal agenesis is often defined as the failure of one or both kidneys to develop. Because penetration of the ureteric bud into the metanephros is the stimulation for the formation of nephrons, true renal agenesis is not associated with the presence of a ureter.23 If the kidney is absent but the ureter is present, the condition is termed renal dysgenesis. Renal agenesis is a relatively uncommon condition but has been reported in a number of different species.22,66,88,98 The right kidney is more frequently reported to be absent compared with the left.22,88 In the authors’ experience, animals with renal agenesis also have other ipsilateral abnormalities associated with the reproductive tract or limbs. As long as the developed kidney is functioning normally, this condition is generally considered to be an incidental finding, usually identified during routine abdominal imaging or abdominal exploration. Congenital absence of a single kidney is not considered to be a significant factor for survival; however, early neonatal death has been reported in Cavalier King Charles spaniels with bilateral renal agenesis detected on postmortem examination.98
Kidneys
Gross Anatomy
Vessels
Physiology
Urine Formation
Urine Concentration
Healing of the Upper Urinary Tract
Perioperative Management
Diagnostic Tests
Renal Imaging
Survey Radiography
Intravascular Contrast Studies
Name (Trade Name)*
Characteristics†
Iodine (mg/mL)‡
Sodium iothalamate (Conray 400)
Ionic and hypertonic
400
Meglumine iothalamate (Conray 60)
Ionic and hypertonic
282
Meglumine (66%) and sodium (10%) diatrizoate (Renografin, Hypaque)
Ionic and hypertonic
370
Iopamidol (Isovue)
Nonionic and isotonic
200
Iohexol (Omnipaque)
Nonionic and isotonic
240
Pyelography
Ultrasonography
Computed Tomography
Magnetic Resonance Imaging
Scintigraphy
Condition
Dose (mL/min/kg)
Normal dog
>3
Normal cat
>2.5
Subclinical renal insufficiency
1.2–2.5
Clinical renal insufficiency (increased BUN and creatinine values)
<1.0–1.3
Normal kidney with minimal function of contralateral kidney
>1.5
Preoperative Considerations
Abnormalities of the Kidney
Renal Agenesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Kidneys
Only gold members can continue reading. Log In or Register to continue