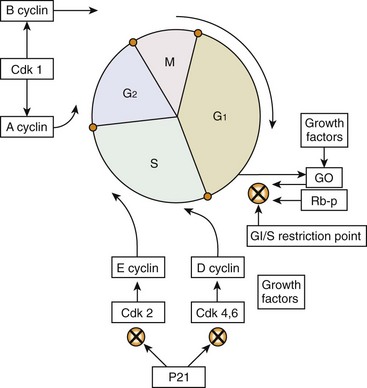
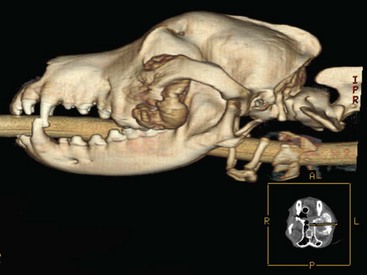
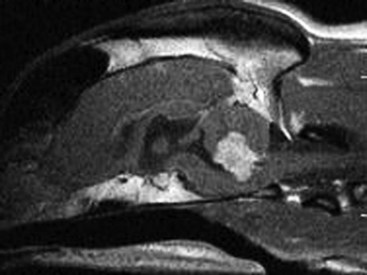
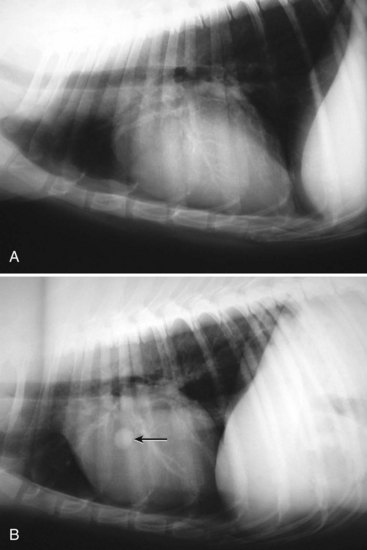
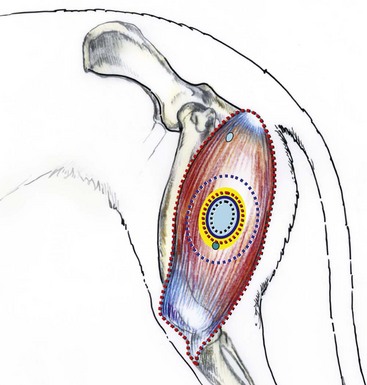
Chapter 25 In human medicine, surgical oncology is a well-established subspecialty. It is impressive that 60% of human patients who are cured of cancer are cured by surgery alone.160 Similarly, in veterinary medicine, surgery is considered the most important component of treatment in dogs and cats suffering from solid tumors. In recent years, veterinary surgical oncology has emerged as a growing specialty in the multidisciplinary care of small-animal cancer patients. The Veterinary Society of Surgical Oncology (VSSO) was established in 2006 to advance the art, science, and practice of surgery for the treatment of cancer in veterinary patients (www.vsso.org). Transformation of a normal cell into a cancerous cell is a complex process of genetic alterations; a complete review of this process is beyond the scope of this chapter. Generally speaking, such alterations result in the activation of tumor-promoting factors via oncogenes or loss of innate tumor inhibitory effects via tumor suppressor genes (e.g., p53, which is also known as the “guardian of the genome”). Oncogenes can be activated by chromosomal translocation, gene amplification, point mutations, and viral insertions.4 Point mutations can be induced by ionizing radiation and chemical carcinogens or may involve mutations in protooncogenes such as K-ras, the epidermal growth factor receptor, and the c-kit growth factor receptor. Genotypic changes then result in phenotypic characteristics that include (1) self-sufficiency in growth signals, (2) insensitivity to antigrowth signals, (3) tissue invasion and metastasis, (4) limitless replicative potential, (5) sustained angiogenesis, and (6) evasion of apoptosis.79 A noted example of tumorigenesis is that of colorectal cancer in human beings with familial adenomatous polyposis. In familial adenomatous polyposis, a mutation on the tumor suppressor gene adenomatous polyposis coli has been identified.129 The adenomatous polyposis coli protein made by the adenomatous polyposis coli gene plays a critical role in several cellular processes. When mutated, the result is an abnormally short and nonfunctional adenomatous polyposis coli protein that cannot suppress cellular overgrowth. This results in a series of events that transform normal colonic epithelium into a benign polyp and ultimately into to a malignant carcinoma.60 The division of somatic cells (Figure 25-1) begins with interphase, where doubling of genetic material occurs. Interphase consists of the GI, G2, and S phases. GI and G2 serve as checkpoints that ensure normal DNA synthesis and cell division. The critical checkpoint for continued progression of the cell cycle occurs within G1 at a point called the restriction point. The synthesis/doubling of new DNA occurs during the S phase. Interphase is followed by division of the doubled genetic material into two daughter cells, called mitosis (M). Regulation of the entry and progression of the cell cycle occurs via proteins called cyclin-dependent kinases, which are important growth signals in the progression of both normal and neoplastic cells.190 Initiation, promotion, and progression of a cell toward a malignant phenotype constitute a complex multistep and multifactorial process. The terms initiation, promotion, and progression can be applied to genetic factors leading to cancer formation, as well as to chemical carcinogenesis. When used in reference to the genetic factors of cancer formation, initiation first occurs with a heritable mutation, followed by a second mutation that promotes the growth advantage of a mutated cell over surrounding cells.101 The initiation steps of chemical carcinogenesis occur when a cell’s DNA is exposed to a mutagen that causes base alterations in nuclear material. Initiation is considered to be an irreversible step; however, not all initiated cells go on to develop into clinically detectable neoplasia. For chemical carcinogens to cause tumor growth, the initiated cell must be exposed to a promoting agent. Tumor formation does not occur if a promoting agent is exposed to a cell first, followed by the initiating agent. Clinically, initiation and promotion lead to benign lesions such as papillomas or polyps.60 Tumor progression occurs when a tumor gains the ability to invade surrounding tissue, establish new blood vessels (angiogenesis), and metastasize to regional and distant sites. All cancers have some genetic basis, but not all cancers are necessarily heritable. By some estimates, only 0.1% to 10% of human cancers are inherited.202 Cancers well known to have a heritable basis in human beings include retinoblastoma, where a mutation in the tumor suppressor gene Rb leads to bilateral retinal tumors in children.145 The tumor suppressor gene, BRCA, is also well established as a cause of heritable forms of human breast cancer. A recent study documented a four-fold increased risk for breast cancer development in English Springer Spaniels in Sweden exhibiting single nucleotide polymorphisms in the BRCA1 and BRCA2 genes.169 Good data support the heritable risk of lymphoproliferative disease in breeds such as Golden Retrievers.143 Although genetic links and associations have been documented within breeds, true genetic heritability has been established only with osteosarcoma of Scottish Deerhounds158 and renal cystadenocarcinoma and nodular dermatofibrosis in German Shepherd Dogs. In renal cystadenocarcinoma and nodular dermatofibrosis, an autosomal dominant pattern of inheritance is found, and the putative renal cystadenocarcinoma and nodular dermatofibrosis gene in dogs appears to be similar to the Birt-Hogg-Dube gene in human beings who exhibit a similar syndrome.92 Viral agents, bacteria, and parasitic organisms all have been implicated in cancer formation. In veterinary medicine, the link between the retrovirus, feline leukemia virus (FeLV), and lymphoproliferative disease is well established. Another retrovirus, the feline sarcoma virus, is a rare cause of fibrosarcoma in cats and requires that a cat be co-infected with the FeLV for carcinogenesis to occur.51 The papilloma virus is a DNA virus that leads to the formation of papillomas in young dogs and other immunocompromised patients. In rare cases, papilloma virus infection can also lead to the formation of squamous cell carcinoma.24,192 Spirocerca lupi, an esophageal parasite, is well documented in endemic areas as the causative agent of various esophageal sarcomas in dogs.140 Last, transmissible venereal tumor is a contagious venereal tumor that is caused by direct cellular transmission (i.e., the tumor cell itself is the causative agent of disease).146,165,203 Asbestos-related mesothelioma is a well-documented tumor of human beings that is caused by a physical carcinogen. Asbestos, a naturally occurring silica-based fiber, was previously used for its fire retardant and insulating properties and has been linked to the formation of canine mesothelioma.75,80 A well-documented example of physical carcinogenesis in veterinary medicine is the formation of injection site sarcomas in cats. Aluminum-based adjuvant in vaccines is thought to be the putative agent for many of these sarcomas; however, the innate biology of cats likely predisposes them to sarcoma formation, as evidenced by ocular sarcomas that occur post trauma and a more recent report of microchip implantation–associated fibrosarcoma.38 The metallurgy of tibial osteotomy plates has been discussed in a case report of canine osteosarcoma associated with tibial plateau leveling osteotomy.22 Ultraviolet carcinogenesis occurs most often in patients with lightly pigmented skin; it occurs with conditions such as squamous cell carcinoma of the eyelids and nasal planum, and on the pinnae of white or lightly pigmented cats. Cutaneous hemangiomas are also reported to occur in areas of chronic ultraviolet exposure in dogs with lightly pigmented skin, such as Whippets.81 In contrast to human beings, no correlation is known between ultraviolet exposure and the formation of cutaneous melanoma in dogs and cats. Experimental data reveal that ultraviolet light in the 290 to 320 nm wavelength range (ultraviolet-B) is responsible for the majority of ultraviolet-induced damage. Ultraviolet irradiation damages the basal cells of skin by causing dimerization of the pyrimidine bases (thymine and cytosine) of DNA.66 Ultraviolet irradiation has also been shown to contribute to tumor formation by suppressing cutaneous T-cell–mediated immunity.188 Ionizing radiation is radiation that produces electromagnetic waves of sufficient energy to cause the ejection of an electron from the atom, resulting in the formation of an ion. Ionizing radiation is an uncommon source of carcinogenesis in human beings and animals, as it requires exposure to a radiation source such as environmental exposure from radon exposure or accidental release from a nuclear reactor, or large doses of radiation from x-rays or a linear accelerator. Tumor formation secondary to previous definitive radiation therapy in veterinary medicine has been documented but is considered to be a rare entity.84 Oncogenes are mutated versions of normal genes that drive the formation of cancer. The normal counterparts of these genes, called protooncogenes, become transformed by one of four mechanisms: (1) retrovirus-mediated transduction, (2) a translocation mutation, (3) amplification, or (4) proviral insertion. Once they are transformed, oncogenes behave in a dominant manner over their endogenous cousins. Oncogenes drive the formation of cancer, as their endogenous role is to drive cell signaling, cell/tissue differentiation, and extracellular matrix production, and to control apoptosis. Translation of oncogenes leads to the transcription of key proteins such as (1) growth factors, (2) growth factor receptors, (3) cytoplasmic kinases/Ras, (4) transcription factors, and (5) antiapoptotic proteins.78 Growth factors and their receptors are particularly relevant with the development of tyrosine kinase inhibitors such as imatinib and toceranib. The growth factor, stem cell factor, is the ligand that binds the c-kit growth factor receptor, leading to downstream activity that drives cell proliferation. In neoplasia, such as canine mast cell tumor, internal tandem duplication mutations lead to a constitutively active c-kit receptor tyrosine kinase receptor (i.e., ligand binding by stem cell factor is no longer needed for proliferative activity).48,118 The c-kit receptor falls under a group of growth factor receptors called platelet-derived growth factor receptors (PDGFRs). The hepatocyte growth factor receptor met plays an important role in the highly malignant nature of canine osteosarcoma and is an appealing site for targeted therapies.113,124 Other well-known classes of growth factor receptors include fibroblast-derived growth factor receptor (FDGFR), insulin-like growth factor receptor (IGF-1R), vascular endothelial growth factor receptor (VEGFR), and hepatocyte growth factor receptor (HGFR).189 Transcription factors are the downstream targets of the growth factor receptors, the Ras signaling molecules, and cytoplasmic kinases. Examples of transcription factors include the estrogen receptors on the nuclei of breast cells, which play an important role in malignant transformation of breast cancer. Transcription factors traditionally have been more difficult targets for anticancer therapy owing to their subcellular location.39 Nonetheless, progress is being made in this field for several potential targets such as the MYC gene, the JAK-STAT pathway, and histone deacetylases.39 The antiapoptotic proteins are the last category of oncoproteins and a crucial component for the normal attrition of cells that have reached the end of their useful life. The oncogene Bcl-2 is a prosurvival molecule that normal regulates entry of a cell into apoptosis. It is often overexpressed in various tumors, leading to continued cell growth and proliferation.156 Oncogenes have a stimulatory effect on cancer growth, whereas the function of the tumor suppressor gene is to counteract the formation of cancer. Mutations in oncogenes are dominant and are considered gain of function mutations; those in tumor suppressor genes are regarded as recessive/loss of function mutations. For these mutations to occur, both alleles need to be damaged, unlike oncogenes, for which only one allele needs to be affected. Discovery of this process led to the development of Knudson’s “two-hit hypothesis.”101 The first mutation in tumor suppressor genes occurs in the germ line (heritable), and a second hit occurs later on. Another important concept in the understanding of tumor suppressor genes is that of loss of heterozygosity. The second hit in Knudson’s hypothesis does not have to be an exogenous carcinogen such as ultraviolet light or radiation. It can occur simply by replacement of the wild-type allele by a mutated version via DNA recombination, a deletion event, or a point mutation. Tumor suppressor genes can be divided into two groups: the gatekeepers and the caretakers.202 Gatekeepers function to inhibit growth while promoting cell death. The biologic function of the caretaker genes is to ensure DNA repair while maintaining genomic stability. The best known tumor suppressor gene is the gatekeeper gene, p53, which is one of the most common mutations found in cancer cells. p53 is crucial for normal cell cycle regulation and serves as a checkpoint for entry into apoptosis. Mutations of p53 are common in canine tumors, including mammary tumor, lymphoma, and osteosarcoma. These mutations have also been identified as a prognostic factor in canine osteosarcoma. Dogs with p53 mutations were reported in one study to have a survival time of 81 days, as opposed to 256 days for dogs without the mutation.98 Mutations in p53 have also been found to be negative prognostic factors in canine mammary tumors.112,204 Certain tumors undergo an orderly progression from a normal cell to one that displays dysplastic change, and finally to a true neoplastic cell. Examples include melanomas and epithelial neoplasms of the gastrointestinal and urogenital/reproductive tracts. For other tumor classes, such as round cell tumors and mesenchymal neoplasms, such orderly progression is not known to occur. In veterinary medicine, the best examples of tumors resulting from an orderly neoplastic progression include ultraviolet (actinic) induced squamous cell carcinoma of the nasal planum, eyelids, and ears of cats, and transitional cell carcinoma of the bladder. Dysplasia is the first microscopically recognizable change in a cell. Characteristics indicative of dysplastic change include anisocytosis, anisokaryosis, mitotic figures, and chromatin changes. The next step in progression is conversion to carcinoma in situ. With this change, transformed cells exhibit a greater number of criteria of malignancy and occupy the entire thickness of the epithelium but have not yet invaded past the basement membrane. Once cells invade past the basement membrane, the cancer is considered to be invasive with the potential for further local invasion, as well as metastasis to distant sites.115 Telomeres are highly conserved nucleoprotein complexes at the ends of linear chromosomes that play crucial roles in aging and cancer. All vertebrate telomeres studied to date possess the (TTAGGG)n nucleotide repeat sequence. Without some means of compensation, 50 to 200 bp of telomeric DNA is lost with each round of replication owing to the inability of DNA polymerase to completely replicate lagging strand DNA, the so-called end replication problem. Telomere attrition in somatic tissues is in essence a tumor suppressor mechanism, as it facilitates elimination of cells that have replicated many times, increasing their likelihood of accumulating deleterious mutations. Telomerase is a highly specialized ribonucleoprotein complex that catalyzes the addition of telomeric sequences onto the 3′ends of chromosomes de novo. Both human and canine neoplastic tissues have been shown to exhibit high levels of telomerase activity. Eighty-five percent of human solid tumors express telomerase, and 92% to 95% of canine tumors have been shown to be telomerase positive. With the exception of stem cells, lens tissue, male germline cells and activated lymphocytes, and canine and human somatic tissues do not express telomerase. This specificity to neoplastic tissues makes telomerase an attractive target for therapeutic intervention, and several anti-telomerase clinical trials are under way in human oncology.103,147,150 Apoptosis, or programmed cell death, is an important homeostatic mechanism that removes cells that have been damaged or have reached the end of their useful life. Lack of apoptosis is considered a hallmark of carcinogenesis and a key characteristic of cancer cells. Apoptosis is associated with distinct morphologic changes, including membrane blebbing, contraction of the cytoplasm, and nuclear condensation. Necrotic cells, by comparison, are characterized by cellular swelling and rupture of the cell membranes. Necrosis is a passive process, and apoptosis is an active one, wherein the cell contributes factors that lead to its own death.104 Cells are triggered to undergo apoptosis when exposed to endogenous signals, as well as external stimuli such as radiation, ultraviolet damage, or exposure to chemotherapeutics. It can also occur with anoikis, whereby a group of cells lose cellular contact with the surrounding stroma/matrix. Entry into apoptosis depends on the balance of antiapoptotic proteins such as the oncogene Bcl-2, and proapoptotic proteins such as the caspases and the tumor suppressor gene, p53.78 Knowledge of the basic origins of cancer and the progression of the neoplastic condition will enable the oncologic surgeon to properly educate clients and understand the rationale for the adjuvant use of certain antitumor agents (e.g., inhibitors of the receptor tyrosine kinase kit).207 It is essential for the oncologic surgeon to be familiar with the disease patterns of commonly seen cancers, in particular, the propensity for local tissue invasion, regional metastasis (i.e., to lymph nodes), distant metastasis, and paraneoplastic syndromes. Three major routes of metastasis are known: (1) hematogenous, (2) lymphatic, and (3) direct seeding. In general, carcinomas and round cell tumors metastasize via lymphatics, and sarcomas metastasize via the hematogenous route.82 The metastatic cascade is a series of events that takes place during the journey of a tumor cell to a distant anatomic site. In broad terms, the process of spread to a distant site involves detachment from the primary tumor, migration through surrounding normal tissues, intravasation into a microvessel, survival of circulation, attachment to a distant endothelial cell (e.g., lung capillary bed), extravasation, proliferation, angiogenesis, and proliferation.25 The process of metastasis, although complex, follows a well-laid path. The tumor cell first must leave the primary disease site by invading past the basement membrane and going in between endothelial cells to reach a capillary bed. One crucial component is the matrix metalloproteinases, which are enzymes that aid in the degradation of extracellular matrix. Intravasation is the entry of tumor cells into a vascular or lymphatic vessel. Because they have now detached from the primary disease site and supportive stroma/matrix, they need to be able to survive the relatively higher shear forces in vessels, evade the host immune system, and resist apoptotic death via anoikis. The cell has to extravasate from the vessel and implant in a distant site, often with a very different tumor microenvironment and with stroma different from its original site. Cells may lay dormant at the site of implantation for weeks to months to years before they are reactivated. The ability of a cell to proliferate in this new environment depends on its ability to develop a new blood supply (angiogenesis), recruit a supporting stroma/extracellular matrix, and produce the necessary growth factors. Angiogenesis is crucial to the survival and proliferative ability of a metastatic cell. Stimulation of this process results from progressive hypoxia that occurs as the metastatic cell proliferates in its new environment. Studies have shown that tumor cells need to be within a 100 to 200 µM distance from a capillary bed for continued growth and the removal of waste products. Hypoxia leads to activation of hypoxia-induced factor (HIF-1a), which is an oxygen-dependent transcription factor. HIF-1a induces the transcription of tumor-derived growth factors such as vascular endothelial growth factor (VEGF).47 Other growth factors liberated from tumor cells include basic fibroblast growth factor (BFGF), hepatocyte-GF (HGF), and platelet-derived growth factor (PDGF). These factors all lead to endothelial cell recruitment and their eventual organization into capillary tubes and vessels.61,174 It should be noted that not all cells that gain access to the circulation are successful in this journey. Metastatic patterns cannot be explained solely on the basis of anatomic factors.82,155 This observation was first made by Dr. Stephen Paget after postmortem examination of more than 700 women with metastatic breast cancer.83,155 Surprising findings revealed that the liver and not the lungs was the most common site of metastasis. Because breast carcinoma cells must travel through the microcirculation of the lungs before arriving at the liver, Dr. Paget deduced that metastatic colonization of another organ did not appear to be merely a result of tumor cells arresting in the first microcirculatory bed they encountered. His observations gave rise to the “seed and soil” hypothesis, whereby tumors cells developed in “fertile soil” or tissues. We now know that this process is a complex set of events that involve tissue-specific growth factors and specific adhesion molecules that allow tumor cells to bind to endothelial cells.82,155 A paradox exists with the simultaneous acceptance of the concept of cancer cell heterogeneity within tumors and the idea that cancer is a clonal process. The cancer stem cell theory accounts for this paradox and states that cells exist “…within a tumor that possess the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor.”31 Cancer stem cell candidates were first identified in acute myelogenous leukemia in human beings and later in solid tumors such as breast, brain, and pancreatic tumors. They have also been identified recently in canine osteosarcoma.210 It is believed that cancer stem cells are responsible for resistance to chemotherapy and radiation therapy by providing a reservoir of cells for tumor repopulation. The cancer stem cell theory is likely best reflected in veterinary oncology in high-grade solid tumors such as mammary carcinoma, hemangiosarcoma, soft tissue sarcoma, and osteosarcoma. It is recognized that these diseases often result in metastatic or recurrent disease, even in the setting of adequate local control and adjuvant chemotherapy. If the stem cell theory holds true, novel therapeutics will have to be designed to address this population of cells that lead to the recurrence of disease or late metastasis. Surgical oncology is a discipline that is reliant on excellent communication skills with clients and referring veterinarians. The first issue concerning client communication involves delivering the difficult news that the pet has cancer, especially with all the negative connotations associated with cancer and death. Clinicians need to be cognizant of the owner’s feelings and emotional state, and should present the news with sympathy, understanding, and honesty.171 Other useful recommendations include the use of empathic expression, active listening, and a variety of media (examples include literature articles, images of postoperative appearance, and videos showing return to function following amputation and major jaw resection) when information is discussed with owners, including prompt sheets and online resources.171 Some institutions and practices have on-site counselors to facilitate these conversations with owners, particularly owners who are having a difficult time in coping with the diagnosis of cancer, need assistance in the decision-making process, or require support during treatment of their pet, or to identify owners who may be suicidal. When communicating with owners, it is important that the clinician clearly communicate and confirm that the information presented has been comprehended adequately. Some owners may have unrealistic expectations of longevity and cure following treatment. In human oncology, patient understanding and awareness of the information presented are often limited, and misunderstandings about prognosis, chance of cure, expected survival, and aims of treatment are frequent.171 Similar limitations in veterinary medicine are understandable, given that many pet owners are trying to cope with the news that their pet has cancer, and then are expected to process all the information regarding cancer type, treatment options, and prognosis. Hence it is important that the clinician ensure that the owner understands the information; it may be helpful for the clinician to schedule a second appointment to discuss treatment options and prognosis once the owner has processed the news regarding the pet’s diagnosis. When choosing a biopsy technique for tumor diagnosis, consideration should be given to the invasiveness of the procedure and the potential for causing hemorrhage into a body cavity (e.g., a large, cavernous splenic mass) or the seeding of tumor cells72 into a body cavity or along a needle tract (e.g., transitional cell carcinoma of the urinary bladder). If the need for surgery is clear, as with a staged-clean cavitated splenic mass that is actively hemorrhaging, it may be in the patient’s best interest to forego diagnostic efforts. The surgeon must decide whether the risks of the diagnostic tests are justified. Incisional biopsy is the removal of a portion of a tumor by sharp incision. This technique is usually performed in cases in which knowing the specific behavior of a tumor may affect an owner’s willingness to treat the pet surgically, or when knowing the identity of the tumor would alter the treatment plan. Biopsy techniques and instrumentation are discussed elsewhere (see Chapter 21). In contrast, excisional biopsy is the removal of the entire tumor along with a surrounding barrier of normal tissue. The main advantage of this technique is that biopsy and gross tumor removal are performed in a single procedure. The primary disadvantage is that if the tumor is highly invasive and the surgeon does not know the identity of the tumor, the level of surgical aggressiveness may be inadequate for complete excision. Such an excision has been termed an unplanned excision.70,162 Cytology findings and anatomic location are important factors when deciding which approach to employ. For example, cytology results may be diagnostic for mast cell tumor, obviating the need for a tissue biopsy. Tumors in the flank region may allow an excisional biopsy to be performed with wide (i.e., 3 cm) margins and a deep fascial plane, but the same approach on a distal extremity may result in a large open wound that will not allow primary closure. Other instances in which excisional biopsy is typically performed include intrathoracic and intra-abdominal masses, because the invasiveness of the biopsy procedure may involve too much risk or may equal the morbidity of the definitive resection if an open biopsy is performed. Ultrasonography is a useful and cost-effective tool for the evaluation of intra-abdominal neoplasia, particularly hepatic, adrenal, and urogenital tumors, and sublumbar node metastasis. Ultrasound can also be used to accurately guide needles and needle-core biopsy instruments for relatively noninvasive tissue sampling. Newer developments in ultrasound technology that have proven useful in the evaluation of primary tumors include Doppler to assess tumor vascularity (e.g., thyroid carcinomas in dogs) and contrast-enhanced harmonic ultrasound for differentiation of benign and malignant hepatic and splenic tumors.149 As an example of the benefits of ultrasonography in surgical planning, ultrasound can be used to differentiate isolated from invasive adrenal tumors.86 Anesthetic and surgical planning for removal of an isolated adrenal tumor is less complicated than an adrenalectomy with en bloc thrombectomy via a partial cavotomy.107 Nuclear scintigraphy provides valuable information in the diagnostic workup of certain tumor types. For instance, whole-body bone scans using radiolabeled technetium-99m hydromethylene diphosphate are particularly useful for the detection of asymptomatic synchronous or metastatic osteosarcoma lesions in other bones. In one study, 7.8% of 399 dogs with appendicular osteosarcoma were diagnosed with a second asymptomatic lesion using nuclear scintigraphy.88 This has important implications for case management, as these dogs are not considered to be good candidates for limb amputation because of the risk of pathologic fracture and catastrophic failure through the second lesion. Bone scans can also be used to assess surgical margins for dogs undergoing limb-sparing surgery.113 Other indications for nuclear scintigraphy include glomerular filtration rate (with technetium-99m diethylenetriaminepentaacetic acid) scans for evaluation of renal function before planned nephrectomy for primary renal tumors or tumors with secondary renal involvement (e.g., adrenal tumors),161 technetium-99m scans for cats and dogs with functional thyroid tumors to identify ectopic or metastatic disease,161 and somatostatin receptor scans (with indium-111 pentetreotide) to identify primary and metastatic lesions in dogs with functional pancreatic insulinomas.69 Advanced imaging techniques such as CT and MRI have revolutionized the management of animals and human beings with neoplasia. For example, before the advent of these imaging modalities, localization of brain tumors was often based on clinical signs alone, but now CT and MRI are used to provide accurate three-dimensional information on tumor location for stereotactic CT-guided biopsy, radioablation, and surgical resection of brain tumors.111 In general, CT is preferred for evaluation of bones and MRI for soft tissue structures, but considerable overlap has been noted in the information that CT and MRI can provide. Both CT and MRI are valuable for determining the degree of involvement of aggressive cutaneous tumors, such as injection site sarcomas, because the extent of the tumor is often recognized to be far greater with these imaging modalities than with palpation alone. CT scans are recommended for evaluation of primary tumors of the axial skeleton, particularly skull, vertebral, and pelvic tumors, and for evaluation of primary and metastatic intrathoracic tumors (because the quality of MRI is decreased by respiratory motion).111 CT scans are faster than MRI scans, but they have lower contrast resolution and require iodinated contrast agents and ionizing radiation.1 Adjacent muscle/fascial planes can also be seen, and bone involvement evaluated. Reconstructed CT images allow the surgeon to choose the orientation in which to visualize the mass in relation to its normal surrounding tissues, and three-dimensional reconstructions can be manipulated at a computer workstation to help the surgeon envision a surgical plan before the time of surgery with complicated cases (such as large skull-based tumors) (Figure 25-2). Important examples of the use of CT include thoracic wall tumors to determine which ribs need to be resected, oral tumors to assess the amount of bone involvement, and contrast CT for adrenal tumors invading the vena cava and cutaneous or subcutaneous masses to assess adjacent muscle/fascia involvement.206 CT-guided biopsy can aid in making the diagnosis in difficult to access locations (e.g., vertebral body), and CT scans are often used for radiation planning purposes. MRI is preferred for tumors of the central (Figure 25-3) and peripheral nervous system and perhaps intra-abdominal organs.1 Its utility is greatest for determining the proximity of tumors to important neurovascular structures and for assessing soft tissue components and intramedullary involvement of canine osteosarcoma before limb-sparing surgery is performed.32 Short tau inversion recovery (STIR) MRI can be particularly useful for evaluating the extent of nerve involvement with malignant peripheral nerve sheath tumors that involve a nerve plexus or roots at the level of the spinal cord. In addition, MRI has higher soft tissue resolution and does not use iodine-based contrast agents. However, MRI scans take longer to perform, metallic implants are a contraindication, and artifacts are common, particularly with motion.1 Ultrasonography can provide similar information to MRI for localization and surgical planning of intra-abdominal neoplasia, but tumor characteristics on T1- and T2-weighted MRI may provide further information on tumor type.86 Two new exciting areas of tumor imaging are positron emission tomography (PET) and single photon emission computed tomography (SPECT).64 PET scans, which provide in vivo information on biochemical and physiologic processes, such as glucose metabolism, are not widely available in veterinary medicine, but are increasingly used in human oncology for clinical staging of tumors and assessment of response to therapy.89 SPECT uses gamma-ray–emitting radionuclides with a gamma camera that reconstructs images in cross-section, providing improved lesion localization over planar scintigraphy. PET typically utilizes a radiopharmaceutical called F-fluorodeoxyglucose (FDG), which is transported into and trapped inside tumor cells because it is not utilized in the glycolytic pathway. Because tumor cells have a higher uptake of glucose when compared with normal cells, higher signal is detected in the neoplastic tissues.157 Owing to the whole-body nature of the imaging, this technique is particularly useful in assessing for metastatic disease and tumor response to therapy. When coupled with a CT scanner (PET-CT), simultaneous images can be obtained and fused. This imaging combination provides information related to both anatomy and physiology. Limitations do exist, as not all tumors display uptake that differentiates them from surrounding background tissues, positive findings are nonspecific (e.g., inflammation can also lead to increased uptake), and this technique is not readily available to most veterinary institutions. Despite these limitations, PET-CT provides one of the most accurate imaging modalities in oncology.6,126,186,195 Another radiopharmaceutical, [123I]-iodo-L-phenylalanine, has been used experimentally to evaluate for gamma camera imaging in vivo in tumor-bearing athymic mice and in human beings with brain tumors.157 In a recent study, use of this tracer in two dogs with synovial cell sarcoma of the tarsus was detailed. Rapid [123I]-2-iodo-L-phenylalanine tumor accumulation was observed with good tumor-to-background contrast and rapid clearance in these two dogs. It was concluded that this radiopharmaceutical is a promising alternative tumor tracer to overcome the known limitations of 18F-fluorodeoxyglucose, and it has the potential to be used for therapeutic purposes. Combinations of imaging modalities can also be helpful, particularly CT and MRI to maximize bone and soft tissue detail, respectively. Another example includes canine orbital myxosarcomas, for which ultrasound, radiography, and MRI were used together to characterize fluid-filled cavities and extension along fascial planes involving the temporomandibular joint.40 Three-view thoracic radiographs are commonly used in the clinical staging of animals with cancer to evaluate for the presence of pulmonary metastasis. Right and left lateral thoracic projections are necessary, as increased perfusion and atelectasis in dependent lung fields result in poor contrast with metastatic lesions, which also have a soft tissue density (Figure 25-4, A, and Figure 25-4, B). Nondependent lung fields have better ventilation, which, combined with greater lesion-to-film magnification, increases the possibility of detecting metastatic lesions. Using high-detail screens, metastatic lesions as small as 6 mm can be detected on thoracic radiographs.42 Thoracic CT scans are significantly more sensitive than radiographs for the detection of metastatic lesions and can detect lesions as small as 1 mm.153 Imaging modalities used to assess for the presence of metastatic disease are tailored to the tumor type. For example, three-view thoracic radiographs are indicated for pulmonary metastasis in a wide range of tumors, particularly visceral hemangiosarcoma and appendicular osteosarcoma,42,125 but not for canine mast cell tumor, as pulmonary metastases are very rare in dogs with mast cell tumor.193 However, abdominal ultrasound and guided aspirates of the spleen and liver are recommended to evaluate for systemic mastocytosis in dogs with high-grade mast cell tumor and for metastasis in dogs with histiocytic sarcoma.193 Nuclear scintigraphy is used to assess for bone metastasis in dogs with appendicular osteosarcoma.13 Based on these findings, tumors are then clinically staged according to a specific system. The World Health Organization’s tumor-node-metastasis (TNM) staging system is used most frequently in veterinary medicine, where T represents characteristics of the primary tumor, N represents the regional lymph nodes, and M represents distant metastasis.151 Tumor staging should be done in a standardized and reproducible manner, as these staging systems are used to facilitate communication between veterinary oncologists, monitor response to treatment, and perhaps provide prognostic information.71 Other staging systems are occasionally used because of their prognostic importance, such as in splenic hemangiosarcoma, in which stage I tumors (localized) have a significantly better prognosis than stage II (ruptured) or stage III (metastatic) disease.125 Prior to definitive surgical resection, appropriate diagnostic and staging tests should be performed to assess whether the animal is a good anesthetic and surgical candidate, and to determine the appropriate surgical plan and “surgical dose.” Co-morbid conditions, whether they are related to the primary tumor (e.g., vomiting and dehydration secondary to a gastrointestinal tumor) or are unrelated (e.g., renal, hepatic, or cardiac disease), increase the risk of surgical morbidity and mortality and may change both the aggressiveness of the surgery and postoperative management.44,71,183 Chemotherapy, radiation therapy, and/or surgery can be altered, incorporated, or eliminated on the basis of co-morbid conditions.44,71 For example, neurologic disease is a contraindication for limb amputation; hence, limb-sparing surgery may be preferable for a dog with appendicular osteosarcoma and concurrent neurologic disease. For postoperative management, cardiotoxic (e.g., doxorubicin) and nephrotoxic (e.g., cisplatin) chemotherapy agents should not be administered to dogs with preexisting cardiomyopathy and renal disease, respectively. With appropriate preoperative management, co-morbid conditions are not a contraindication to surgery and will reduce the physiologic stresses associated with anesthesia and surgery.183 For instance, anemia is relatively common in animals with cancer and has been reported in 30% to 90% of people with cancer, being more common in advanced disease.100 Anemia may be caused by chronic disease, blood loss, or myelophthisis, and is associated with poorer survival times and local tumor control rates compared with cancer in human patients without anemia.100 Administration of blood products has been recommended to improve oxygen-carrying capacity and to potentially decrease complications associated with hypotension and wound healing.175 However, the necessity and timing of blood transfusions are controversial in human medicine and have not been defined in veterinary medicine. In general, administration of blood products may not be required in animals with chronic anemia, whereas transfusions should be considered in animals with acute intraoperative blood loss (i.e., blood loss greater than 25% of blood volume or packed cell volume less than 20%) and hypotension (mean arterial pressure less than 80 mm Hg, or systolic arterial pressure less than 100 mm Hg).95 Animals should be cross-matched or blood-typed before blood products are administered, to minimize the risk of transfusion reactions.95 Tumor-associated pain is present in 15% of people with nonmetastatic tumor, 33% of those with early metastatic tumor, and up to 100% of individuals with advanced metastatic tumor.135 Pain in these cases occurs because of mechanical or chemical stimulation of nociceptors by the tumor (62% to 78%) or as the result of diagnostic or therapeutic procedures (19% to 25%).135 The deleterious effects of pain on outcome are well documented and can be ameliorated with the administration of analgesic agents (e.g., nonsteroidal antiinflammatory drugs, partial agonist/antagonists, opioids, alpha-2 agonists, N-methyl-D-aspartate antagonists) through local, regional (e.g., epidural), or systemic routes, or by alternative therapies such as acupuncture.135 Enteral nutrition and antibiotic prophylaxis are recommended when appropriate. These are described in detail elsewhere, including indications, techniques, feeding protocols, and complications of enteral nutrition,130 as well as indications, antimicrobial selection, and timing of administration for antibiotic prophylaxis.49 General anesthesia is required for definitive surgical resection, although some tumors in appropriate locations may be amenable to excision using a combination of sedation and local or regional anesthesia. Local anesthetics should not be administered intratumorally because this will distort tumor architecture, will increase the difficulty of histopathologic interpretation, and may potentiate metastasis.183 Depending on the patient’s preoperative condition, the presence of paraneoplastic syndromes, and tumor type and location, the surgeon should be prepared to address possible intraoperative and postoperative complications. Following induction of general anesthesia, preparation of the animal should include a widely clipped area to facilitate wound closure if the aggressiveness of surgery changes, or if reconstructive procedures become necessary to achieve wound closure.44 Gentle skin preparation is important because vigorous scrubbing can result in tumor cell exfoliation and increased risk of metastasis.44,71,183 The surgical principles first described by Dr. William Halsted are important for all surgeries, particularly oncologic surgery, and include gentle handling of tissue, aseptic technique, sharp anatomic dissection of tissue, careful hemostasis, obliteration of dead space, and avoidance of tension.74 Despite the fact that surgical techniques have become more advanced and aggressive, adherence to Halsted’s principles minimizes the risks of postoperative complications and local tumor recurrence. The aggressiveness of surgical resection, or surgical “dose,” is categorized as intralesional (or debulking), marginal, wide, and radical (Figure 25-5). These categories were first proposed for musculoskeletal tumors by Dr. William Enneking, but have since gained wide acceptance for all solid tumors.45 The most common mistake in surgical oncology is to use too low a surgical dose, particularly because of the fear of being unable to close the resultant defect. In human medicine, two surgical teams are sometimes involved in the excision of tumors: one team for surgical resection and another team for subsequent reconstruction, to avoid this situation. Because this is unrealistic in veterinary medicine, the use of sterile surgical markers to delineate margins prior to incision assists in orientating the surgeon and overcoming the subconscious concerns of wound closure. As has been stated previously, “it is better to leave a wound open … than to leave tumor cells remaining.”211
Introduction to Oncologic Surgery for the General Surgeon
The Origin and Progression of Cancer
Cell Biology: Cell Division, Cell Cycle, Cell Death
Initiation, Promotion, Progression to Malignant Phenotype, and Carcinogenesis
Heritable Carcinogenesis
Biologic Carcinogenesis
Physical Carcinogenesis
Ultraviolet Light
Ionizing Radiation
Oncogenes
Tumor Suppressor Genes
Tumor Progression: Healthy, Dysplasia, Carcinoma In Situ, Localized Cancer, Regional Spread, Disseminated
Limitless Replicative Potential
Apoptosis
Patterns of Local Behavior and Metastasis
The Cancer Stem Cell Hypothesis
Client Education and Communication
Patient Assessment and Staging
Approach to Biopsy
Imaging
Ultrasonography
Nuclear Scintigraphy
Computed Tomography and Magnetic Resonance Imaging
Distant Metastasis Staging
Patient Approach
Co-Morbid Conditions
Pain and Analgesia
Other Considerations
Anesthetic Management
Surgical Preparation
Surgical Principles
Margins of Excision
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Introduction to Oncologic Surgery for the General Surgeon
Only gold members can continue reading. Log In or Register to continue