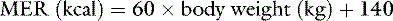Chapter 14 Introduction to Fluid Therapy
Fluid therapy is supportive. The underlying disease process that caused the fluid, electrolyte, and acid-base disturbances in the patient must be diagnosed and treated appropriately. Normal homeostatic mechanisms allow the clinician considerable margin for error in fluid therapy, provided that the heart and kidneys are normal. This is fortunate because estimation of the patient’s fluid deficit is difficult and may be quite inaccurate. The purpose of this chapter is to provide an overview of the principles of fluid therapy. The composition and distribution of body fluids are discussed in Chapter 1, and the technical aspects of vascular access are discussed in Chapter 15. Fluid therapy potentially consists of three phases: resuscitation, rehydration, and maintenance. Most patients in shock (see Chapter 23) require rapid administration of a large volume of crystalloid, colloid, or other fluid to expand the intravascular space and correct perfusion deficits. Dehydrated patients also require sustained administration of crystalloid fluids for 12 to 36 hours to replace fluid losses from the interstitial and intracellular spaces. Patients with normal hydration unable to consume sufficient water to sustain fluid balance require maintenance fluid therapy with crystalloid solutions. In formulating and implementing a fluid therapy plan, eight questions should be considered10,28:
1. Is the patient suffering from a shock syndrome that requires immediate fluid administration?
3. Can the patient consume an adequate volume of water to sustain normal fluid balance?
4. What type of fluid should be given?
5. By what route should the fluid be given?
6. How rapidly should the fluid be given?
7. How much fluid should be given?
Is the patient suffering from a shock syndrome that requires immediate fluid administration?
Shock patients (see Chapter 23) urgently require fluid therapy. The presence of altered mental status and cool extremities in association with tachycardia or severe bradycardia, mucous membrane pallor, prolonged or absent capillary refill time, reduced or absent peripheral pulses, and hypotension are among the most common physical examination findings in patients in shock. Such physical examination findings in association with a compatible clinical history are the basis for the decision to institute a resuscitation phase of fluid therapy. Some forms of shock may be associated with variations in these physical examination findings, and it is crucial to understand the different shock syndromes. (See Chapter 23 for more information on shock.)
Is the patient dehydrated?
The need for a rehydration phase is dependent on the underlying condition of the patient. For surgical patients, there are additional indications for fluid therapy, such as maintenance of venous access for emergencies and establishment of diuresis to maintain renal perfusion during anesthesia (see Chapter 17). For medical patients, the answer to this question depends on an assessment of the animal’s state of hydration. The hydration status of the animal is estimated by careful evaluation of the history, physical examination findings, and the results of a few simple laboratory tests.7,11
In its most narrow sense, dehydration refers to loss of pure water. However, the term dehydration usually is used to include hypotonic, isotonic, and hypertonic fluid losses. The type of dehydration is classified by the tonicity of the fluid remaining in the body (e.g., a hypotonic loss would result in hypertonic dehydration). Isotonic and hypotonic losses are most common in small animal practice. Isotonic fluid loss can result in volume depletion and nonosmotic stimulation of antidiuretic hormone (ADH) release, thus preventing effective excretion of consumed water and resulting in hypotonic dehydration. Types of dehydration are depicted in Figure 3-1 and are discussed in detail in Chapter 3.
Fluid balance
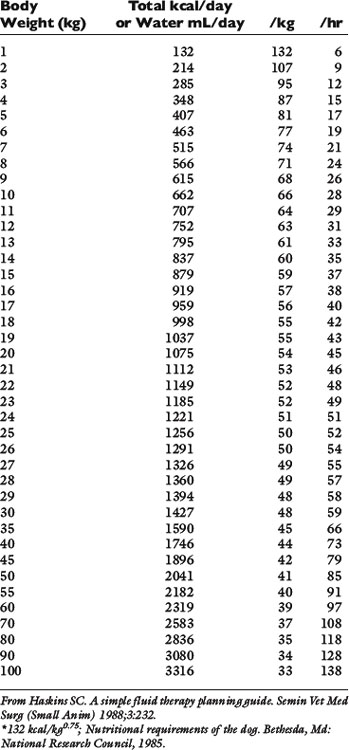
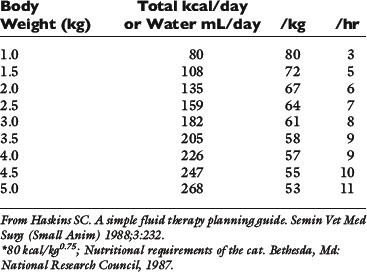
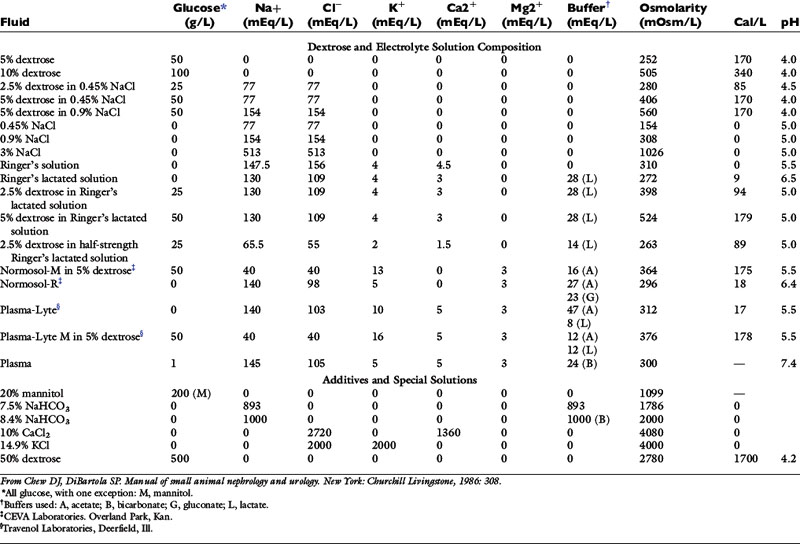
Normal sources of fluid input are water consumed in food, water that is drunk, and water produced in the body as a result of metabolism. Nutrient oxidation produces approximately 0.1 g of water per kilocalorie of energy released.2 Maintenance water and electrolyte needs parallel caloric expenditure,20,22,23 and normal daily losses of water and electrolytes include respiratory, fecal, and urinary losses. Estimated daily caloric and water requirements for dogs and cats are shown in Tables 14-1 and 14-223 and in Figure 14-1.20 Respiratory loss of fluid can be important in dogs because panting has been adapted for thermoregulation in this species. Pyrexic patients also can lose fluid by this route. Normally, cutaneous losses are unimportant in dogs and cats because eccrine sweat glands are limited to the foot pads and do not play an important role in thermoregulation in these species. Sympathetic stimulation as a result of heat stress in the cat may result in increased secretion of saliva, and a small volume of fluid may be lost by this route.
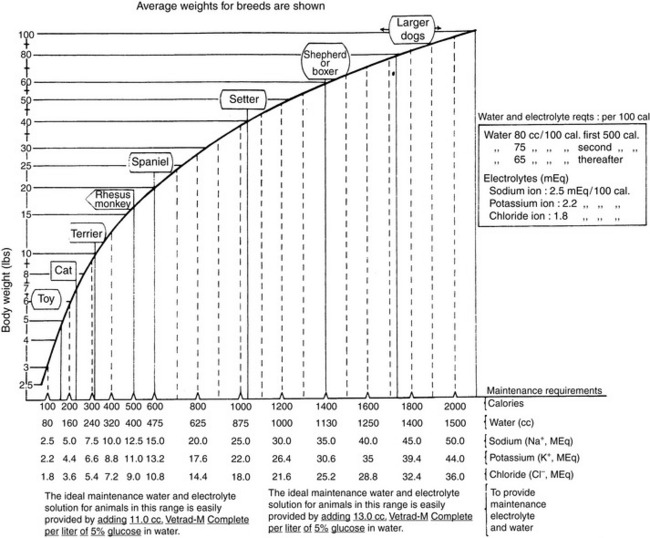
Figure 14-1 Daily water, calorie, and electrolyte requirements for dogs and cats.
(From Harrison JB, Sussman HH, Pickering DE. Fluid and electrolyte therapy in small animals. J Am Vet Med Assoc 1960;137:638.)
History
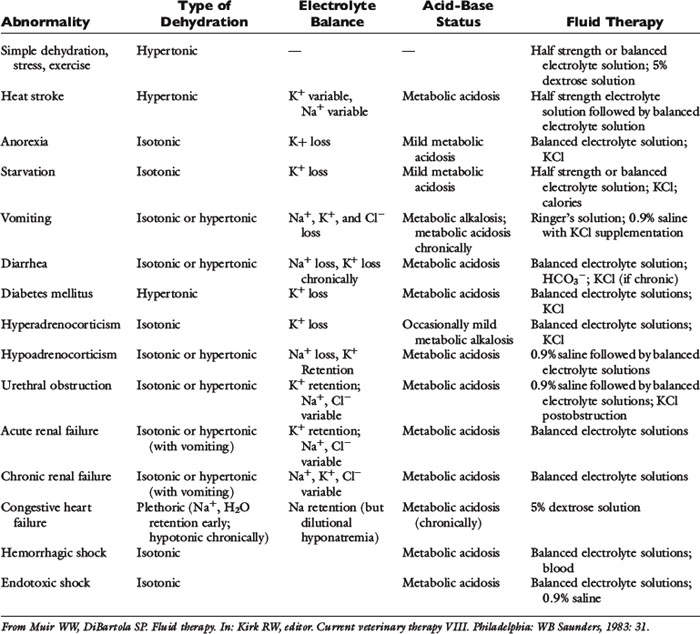
Historical information about the route of fluid loss may suggest the affected fluid compartment or compartments, as well as the patient’s electrolyte and acid-base derangements. The time period over which fluid losses have occurred and an estimate of their magnitude should be determined. Information about food and water consumption, gastrointestinal losses (e.g., vomiting, diarrhea), urinary losses (i.e., polyuria), and traumatic losses (e.g., blood loss, extensive burns) should be obtained from the owner. Excessive insensible water losses (e.g., increased panting, pyrexia) and third-space losses may be determined from the history and physical examination. In addition, the clinician’s knowledge of the suspected disease can aid in predicting the composition of the fluid lost (e.g., vomiting caused by pyloric obstruction leads to loss of hydrogen, chloride, potassium, and sodium ions and development of metabolic alkalosis, whereas small bowel diarrhea typically leads to loss of bicarbonate, chloride, sodium, and potassium ions and development of metabolic acidosis) (Table 14-3).
Physical examination
The physical findings associated with fluid losses of 5% to 15% of body weight vary from no clinically detectable changes (5%) to signs of hypovolemic shock and impending death (15%) (Table 14-4).7,11,20 The clinician may estimate the hydration deficit by evaluating skin turgor or pliability, the moistness of the mucous membranes, the position of the eyes in their orbits, heart rate, character of peripheral pulses, capillary refill time, and extent of peripheral venous distention (e.g., inspection of jugular veins). A decrease in the volume of the interstitial compartment leads to decreased skin turgor and dryness of the mucous membranes. A decrease in plasma volume leads to tachycardia, alterations in peripheral pulses, and collapse of peripheral veins. When these cardiovascular signs are present, the patient is in shock and should be resuscitated promptly before correction of the hydration deficit. Typically, such signs of hypovolemic shock appear with loss of at least 10% to 12% of the patient’s body weight. The fluid deficit in a given patient is difficult to determine with accuracy because of the subjectivity of skin turgor evaluation and the possibility of undetected ongoing (contemporary) losses. Thus a crude clinical estimate of hydration status and the patient’s response to fluid administration become important tools in evaluating the extent of dehydration that was present and in formulating ongoing fluid therapy.
Table 14-4 Physical Findings in Dehydration
| Percent Dehydration | Clinical Signs |
|---|---|
| <5 | Not detectable |
| 5-6 | Subtle loss of skin elasticity |
| 6-8 | Definite delay in return of skin to normal position |
| Slight prolongation of capillary refill time | |
| Eyes possibly sunken in orbits | |
| Possibly dry mucous membranes | |
| 10-12 | Tented skin stands in place |
| Definite prolongation of capillary refill time | |
| Eyes sunken in orbits | |
| Dry mucous membranes | |
| Possibly signs of shock (tachycardia, cool extremities, rapid and weak pulses) | |
| 12-15 | Definite signs of shock |
| Death imminent |
From Muir WW, DiBartola SP. Fluid therapy. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders, 1983: 33.
Skin turgor is dependent on the amount of subcutaneous fat and elastin and on interstitial volume. Detection of dehydration by skin turgor is dependent on the animal’s skin turgor before dehydration developed, the position of the animal (e.g., standing, recumbent) when the skin is checked, the site used for evaluation, and the amount of subcutaneous fat.19 Skin pliability should be tested over the lumbar region with the dog in a standing position. When evaluated by skin turgor, obese animals may appear well hydrated owing to excessive subcutaneous fat despite being dehydrated. Conversely, emaciated animals and older animals may appear more dehydrated than they actually are because of lack of subcutaneous fat and elastin. A false impression of dehydration also may occur with persistent panting, which may dry the oral mucous membranes. The urinary bladder should be small in a dehydrated animal with normal renal function. A large, urine-filled bladder in a severely dehydrated patient indicates failure of the normal renal concentrating mechanism.
Body weight recorded on a serial basis traditionally has been thought to be the best indicator of hydration status, especially when fluid loss has been acute and previous body weight has been recorded. Loss of 1 kg of body weight indicates a fluid deficit of 1 L. Unfortunately, previous body weight is often unknown in animals presented for treatment. However, records from previous routine hospital visits may provide this information. Despite conventional reasoning, clinician estimates of hydration in dogs and cats admitted to a veterinary teaching hospital intensive care unit did not reliably predict changes in weight after 24 to 48 hours of fluid therapy.18 Loss of weight in chronic diseases includes loss of muscle mass and fluid loss. An anorexic animal may lose 0.1 to 0.3 kg of body weight per day per 1000 kcal of energy requirement.13 Losses in excess of this amount indicate fluid loss. Another factor that must be considered in evaluating body weight is the possibility of third-space loss. Fluid lost into a third space does not decrease body weight.23
Laboratory findings
The hematocrit or packed cell volume (PCV), total plasma protein concentration (TPP), and urine specific gravity (USG) are simple laboratory tests that can aid in the evaluation of hydration. It is important to obtain these values before initiating fluid therapy. The PCV and TPP should be evaluated together to minimize errors in interpretation. The PCV and TPP increase with all types of fluid losses excluding hemorrhage, whereas serum sodium concentration increases, decreases, or remains unchanged depending on the loss (e.g., hypotonic, hypertonic, isotonic). The effects of the different types of dehydration on the serum sodium concentration are discussed in Chapter 3. Table 14-5 shows possible interpretations of various combinations of PCV and TPP values. The PCV alone may be an unreliable indicator of hemoconcentration in water-deprived dogs, and although TPP increases, test results may not be above the upper limit of the normal range.19 In one study of dogs and cats admitted to an intensive care unit, baseline measurements of PCV and TPP were not abnormally high in animals judged clinically to be dehydrated, and fluid therapy with crystalloids in dogs had no significant effect on PCV, although TPP decreased slightly.18 The USG before fluid therapy is helpful in the preliminary evaluation of renal function. USG should be high (>1.045) in a dehydrated dog or cat if renal function is normal. This may not be true if other disorders affecting renal concentrating ability, such as medullary washout of solute, are present. Furthermore, previous administration of corticosteroids or furosemide can decrease urinary concentrating ability. After fluid therapy has been initiated, USG falls into the isosthenuric range if rehydration has been achieved.
Table 14-5 Interpretation of Hematocrit and Total Plasma Protein Concentrations
| PCV (%) | Total Plasma Proteins (g/dL) | Interpretation |
|---|---|---|
| Increased | Increased | Dehydration |
| Increased | Normal or decreased | Splenic contraction |
| Polycythemia | ||
| Dehydration with preexisting hypoproteinemia | ||
| Normal | Increased | Normal hydration with hyperproteinemia |
| Anemia with dehydration | ||
| Decreased | Increased | Anemia with dehydration |
| Anemia with preexisting hyperproteinemia | ||
| Decreased | Normal | Nonblood loss anemia with normal hydration |
| Normal | Normal | Normal hydration |
| Dehydration with preexisting anemia and hypoproteinemia | ||
| Acute hemorrhage | ||
| Dehydration with secondary compartment shift | ||
| Decreased | Decreased | Blood loss |
| Anemia and hypoproteinemia | ||
| Overhydration |
From Muir WW, DiBartola SP. Fluid therapy. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders, 1983: 34.
What type of fluid should be given?
Some types of colloids may be used in patients with shock and in those with severe hypoalbuminemia (i.e., albumin <1.5 g/dL). A major limitation to the use of plasma as a colloid is the rapid disappearance of albumin from the vascular space. Dextran 70 is a polymer of glucose that has an average molecular weight of 70,000. Its use in humans has been associated with coagulopathies. Hetastarch has an average molecular weight of 480,000. In humans, coagulopathies also have been associated with the use of hetastarch but typically only when standard dosage recommendations have been exceeded. The main advantages of colloids are that more of the administered solution remains in the plasma compartment and there generally is thought to be less risk of edema in patients with an intact endothelium. Colloids are discussed in detail in Chapter 27.
Crystalloid solutions are equally effective in expanding the plasma compartment, but 2.5 to 3.0 times as much crystalloid solution must be given (compared with a colloid solution) because the crystalloid is distributed to other sites (e.g., interstitial compartment, intracellular compartment).26,27,39 Pulmonary capillaries normally are more permeable to protein, resulting in a higher interstitial concentration of protein and more resistance to leakage of fluid from capillaries.30 Peripheral edema is more likely to occur after crystalloid administration because muscle and subcutaneous capillaries are less permeable to protein.
Crystalloid solutions also can be classified as replacement or maintenance solutions. The composition of replacement solutions (e.g., lactated Ringer’s, Normosol-R, Plasma-Lyte 148) resembles that of ECF (Figure 14-2). Maintenance solutions (e.g., Normosol-M, Plasma-Lyte 56) contain less sodium (40 to 60 mEq/L) and more potassium (15 to 30 mEq/L) than replacement fluids. A simple maintenance solution can be formulated by mixing one part 0.9% NaCl with two parts 5% dextrose and adding 20 mEq KCl per liter of final solution. The approximate composition of such a fluid would be 51 mEq/L sodium, 20 mEq/L potassium, 71 mEq/L chloride, and 33.5 g/L dextrose. It would provide 133 kcal/L and have an osmolality of 328 mOsm/kg. An alternative maintenance solution may be made by mixing one part lactated Ringer’s solution with two parts 5% dextrose and adding 20 mEq KCl per liter of final solution. This solution has the following approximate composition: 43 mEq/L sodium, 21 mEq/L potassium, 56 mEq/L chloride, 1 mEq/L calcium, 9 mEq/L lactate, and 33.5 g/L dextrose. It would provide 133 kcal/L and have an osmolality of 317 mOsm/kg.
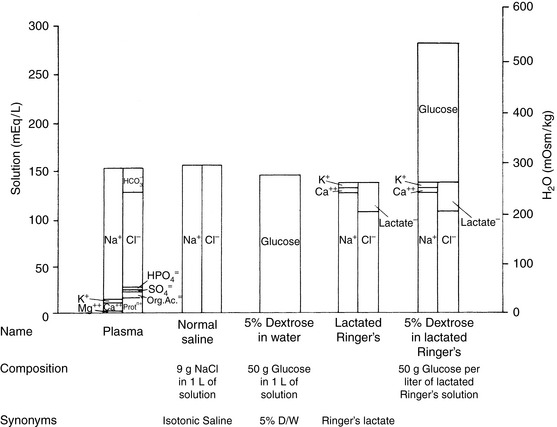
Figure 14-2 Comparison of electrolyte composition of plasma with that of commonly used crystalloid solutions.
(From Muir WW, DiBartola SP. Fluid therapy. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders, 1983: 30.)
The veterinary practitioner can manage most animals requiring fluid therapy with a limited number of crystalloid and additive solutions. The most useful crystalloid solutions for routine use are a balanced replacement solution (e.g., lactated Ringer’s solution, Normosol-R, Plasma-Lyte 148), 0.9% saline, and 5% dextrose in water. The solute composition of these fluids is compared with that of ECF in Figure 14-2, and the electrolyte composition of several commercially available solutions is summarized in Table 14-6.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree