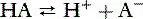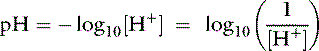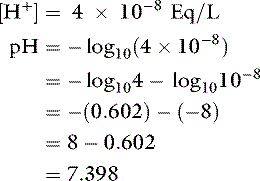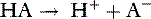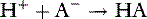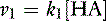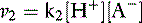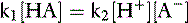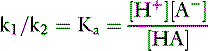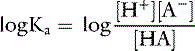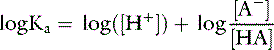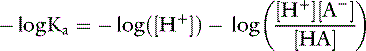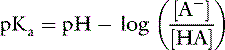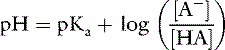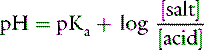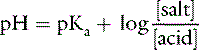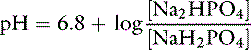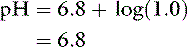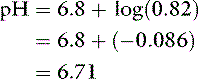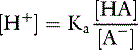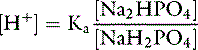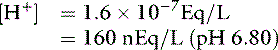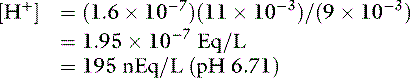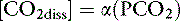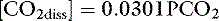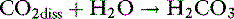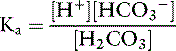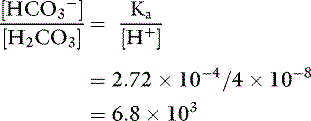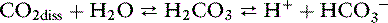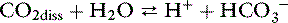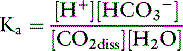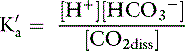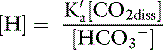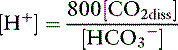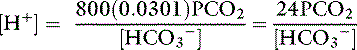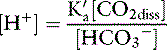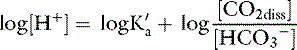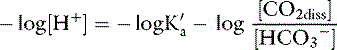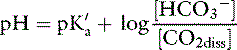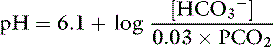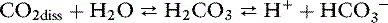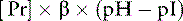Chapter 9 Introduction to Acid-Base Disorders
Concept of pH
Thus, at the normal extracellular fluid (ECF) [H+] of 40 nEq/L (4 × 10−8 Eq/L):
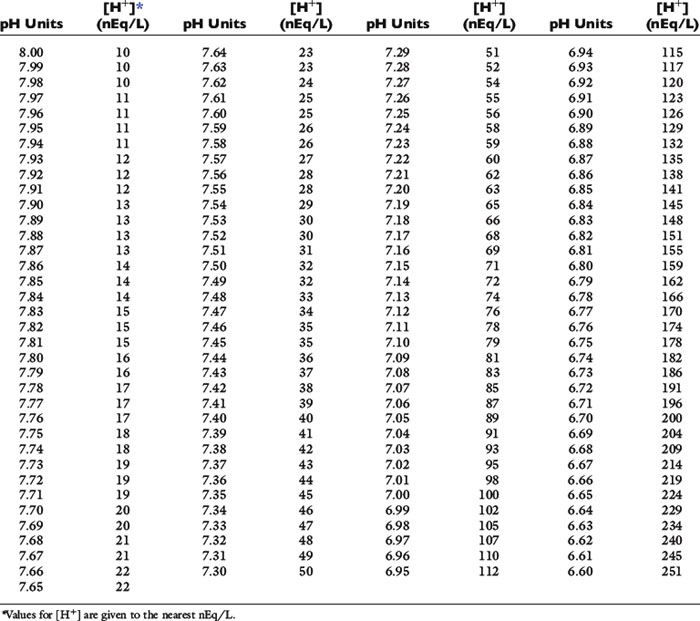
There is an inverse relationship between pH and [H+]: the greater the [H+], the lower the pH. Furthermore, pH and [H+] vary not linearly with one another but exponentially as shown in Figure 9-1. The [H+] for a given pH within the physiologic range is given in Table 9-1.

Figure 9-1 Exponential relationship between [H+] and pH.
(From Madias NE, Cohen JJ. Acid-base chemistry and buffering. In: Cohen JJ, Kassirer JP, editors. Acid-base. Boston: Little, Brown, 1982: 5.)
Law of mass action
The velocity of the first reaction can be written:
and the velocity of the second reaction:
Taking the base 10 logarithm of both sides of the dissociation equilibrium equation yields:
Applying the concept of pH to both the hydrogen ion concentration and dissociation constant, Ka:
Concept of buffering
If the amounts of Na2HPO4 and NaH2PO4 are equal, their ratio is 1.0:
By contrast, an aqueous solution containing 1 mmol/L HCl (10−3Eq/L) would have a pH of 3.0.
By solving the dissociation equilibrium equation for [H+], the same can be shown:
For the previously described solution of sodium phosphate:
After addition of 1 mmol of HCl:
If the amount of strong acid (e.g., HCl) or base (e.g., NaOH) added to a solution of a weak acid and its salt (i.e., a buffer solution) is plotted against pH, the resulting relationship is called a titration or buffer curve (Fig. 9-2). The curve is sigmoidal, and its slope is greatest in the midregion, over which the curve is approximately linear. In the pH range associated with the midregion of the curve, the change in pH is smallest for a given amount of added acid or base and buffer capacity is greatest at the midpoint of the curve. At this point, there are equal amounts of the weak acid and its conjugate salt, and as shown by the Henderson-Hasselbalch equation, pH = pKa. The region of best buffer capacity extends approximately 1.0 pH unit on either side of the pKa. Thus, a buffer is most effective within one pH unit of its pKa. The pKa values for some important biologic compounds are listed in Table 9-2.

Figure 9-2 Titration curve for an aqueous solution containing a phosphate buffer.
(From Ruch TC, Patton HE (eds): Physiology and biophysics, 20th edition, Saunders, Philadelphia, 1974.)
Table 9-2 pKa Values of Biologically Important Compounds*
| Compound | pKa |
|---|---|
| Phosphoric acid | 2.0 |
| Citric acid | 2.9 |
| Carbonic acid (pKa) | 3.6 |
| Acetoacetic acid | 3.6 |
| Lactic acid | 3.9 |
| Citrate1− | 4.3 |
| Acetic acid | 4.6 |
| 3-Hydroxybutyric acid | 4.7 |
| Creatinine | 5.0 |
| Citrate2− | 5.6 |
| Uric acid | 5.8 |
| Organic phosphates | 6.0-7.5 |
| Carbonic acid (pK’a) | 6.1 |
| Imidazole group of histidine | 6.4-7.0 |
| Oxygenated hemoglobin | 6.7 |
| Phosphate1− | 6.8 |
| α-Amino (amino-terminal) | 7.4-7.9 |
| Deoxygenated hemoglobin | 7.9 |
| Ammonium | 9.2 |
| Bicarbonate | 9.8 |
| Phosphate2− | 12.4 |
* Compounds with pKa values in the range of 6.4-8.4 are most useful as buffers in biologic systems. The pKa values for the imidazole group of histidine and for α-amino (amino-terminal) amino groups are for those side groups in proteins. The pKa range for organ phosphates refers to such intracellular compounds as adenosine triphosphate, adenosine diphosphate, and 2,3-diphosphoglycerate.
The bicarbonate–carbonic acid system: physical chemistry
where α is a factor called the solubility coefficient of CO2. The solubility coefficient of CO2 has a value of 0.0301 mmol/L/mm in arterial plasma at 37˚ C. Thus:
Dissolved CO2 combines with water to form carbonic acid:
The uncatalyzed reaction proceeds slowly, but its rate is dramatically increased by the enzyme carbonic anhydrase, which is present in abundance in the body (e.g., red cells, renal tubular cells). In the body, therefore, the hydration of CO2 to form H2CO3 reaches equilibrium almost instantaneously. Normally, the equilibrium is so far to the left that there are approximately 340 molecules of dissolved CO2 for each molecule of carbonic acid.42
The dissociation of carbonic acid can be expressed using the law of mass action:
The reaction of dissolved CO2 in aqueous body fluids can be summarized as:
The law of mass action for this equilibrium can be expressed as:
In body fluids at 37˚ C, K′a is approximately equal to 8 × 10−7 mol/L and p K′a equals 6.1. An approximate value of 6.1 for this p K′a is valid at temperatures ranging from 30° to 40° C (86 to 104° F) and pH values ranging from 7.0 to 7.6.37
Using the solubility coefficient for carbon dioxide yields:
Substituting 6.1 for the value of pK′a and applying the solubility coefficient for CO2, we obtain:
Body buffers
Body buffers can be divided into bicarbonate, which is the primary buffer system of ECF, and nonbicarbonate buffers (e.g., proteins and inorganic and organic phosphates), which constitute the primary intracellular buffer system. Bone is a prominent source of buffer and can contribute calcium carbonate and, to a lesser extent, calcium phosphate during chronic metabolic acidosis. Bone may even account for up to 40% of the buffering of an acute acid load in the dog.9 After administration of NaHCO3, carbonate can be deposited in bone.
Proteins as buffers
Plasma proteins play a limited role in extracellular buffering, whereas intracellular proteins play an important role in the total buffer response of the body. The buffer effect of proteins is the result of their dissociable side groups. For most proteins, including hemoglobin, the most important of these dissociable groups is the imidazole ring of histidine residues (pKa, 6.4 to 7.0). Amino-terminal amino groups (pKa, 7.4 to 7.9) also contribute somewhat to the buffer effect of proteins. Other side groups are relatively unimportant because their pKa values are either too high or too low to be useful in the normal physiologic range of pH. The pKa values for the dissociable groups of proteins are listed in Table 9-3.
Table 9-3 pK′a Values for Dissociable Groups Found in Proteins
| Dissociable Group (Amino Acid) | pK′a |
|---|---|
| α-Carboxyl | 3.6-3.8 |
| β-Carboxyl (aspartic acid) | ≈4.0 |
| γ-Carboxyl (glutamic acid) | ≈4.0 |
| Imidazole (histidine) | 6.4-7.0 |
| α-Amino | 7.4-7.9 |
| Sulfhydryl (cysteine) | ≈9.0 |
| ε-Amino (lysine) | 9.8-10.6 |
| Phenolic (tyrosine) | 8.5-10.9 |
| Guanidino (arginine) | 11.9-13.3 |
From Madias NE, Cohen JJ: Acid-base chemistry and buffering. In Cohen JJ, Kassirer JF, editors: Acid-base, Boston, 1982, Little, Brown & Co., p. 16.
Hemoglobin is responsible for more than 80% of the nonbicarbonate buffering capacity of whole blood, whereas plasma proteins contribute 20%. Of the plasma proteins, albumin is much more important than are the globulins. The buffer value of albumin is 0.12 to 0.14 mmol/g/pH unit, whereas that of globulins is 0 to 0.08 mmol/g/pH unit.38,69,71 The difference results from a larger number of histidine (Fig. 9-3) residues in albumin.

Figure 9-3 The imidazole group of histidine.
(From Madias NE, Cohen JJ. Acid-base chemistry and buffering. In: Cohen JJ, Kassirer JP, editors. Acid-base. Boston: Little, Brown, 1982: 16.)
The isoelectric point (pI) is the pH at which a substance has no tendency to move in an electric field and thus has no net charge. For proteins, this means that the sum of the charges on the negative side groups (e.g., R–COO−) equals the sum of the charges on the positive side groups (e.g., R–NH3+). At physiologic pH (7.4), plasma proteins are polyanions because their pIs range from 5.1 to 5.7. The net negative charge on plasma proteins in mEq/L can be calculated as:38
where [Pr] is the concentration of plasma proteins in grams per liter, β is the buffer value of plasma proteins in millimoles per gram per pH unit, pH is the ECF pH, and pI is the isoelectric point of plasma proteins. Using this formula, it can be calculated that, at a normal plasma protein concentration of 7 g/dL, average buffer value of 0.1 mmol/g/pH unit, and pI range of 5.1 to 5.7, plasma proteins contribute 12 to 16 mEq/L of negative charge. In dogs, the mean contribution of charge by plasma proteins is approximately 16 mEq/L.16,76
Physiologic lines of defense in acid-base disturbances
The volatile acid CO2 cannot be buffered by HCO3−, and the hydrogen ions resulting from the dissociation of carbonic acid must titrate intracellular buffers, such as proteins (especially hemoglobin in red cells) and phosphates. Renal adaptation is characterized by increased HCO3− reabsorption and net acid excretion, mechanisms that require 2 to 5 days to achieve maximal effectiveness. The buffer response of the body to the primary acid-base disorders is considered in more depth in the chapters on those disorders (see Chapters 10 and 11).
Primary acid-base disturbances
Metabolic acidosis is characterized by a decreased plasma HCO3− concentration and decreased pH (increased [H+]) caused by either HCO3− loss or buffering of a noncarbonic (nonvolatile or fixed) acid. Metabolic alkalosis is characterized by an increased plasma HCO3− concentration and increased pH (decreased [H+]), usually caused by a disproportionate loss of chloride ions from the body (i.e., loss of fluid with a chloride concentration greater than that of ECF) or hypoalbuminemia (because albumin is a weak acid). In the absence of volume depletion or renal dysfunction, it is extremely difficult to produce metabolic alkalosis by administration of alkali. Respiratory acidosis is characterized by increased Pco2 (hypercapnia) caused by alveolar hypoventilation. Respiratory alkalosis is characterized by decreased Pco2 caused by alveolar hyperventilation (hypocapnia). In one study, metabolic acidosis was the most common acid-base disturbance encountered in dogs.17
Each primary metabolic or respiratory acid-base disturbance is accompanied by a secondary, or adaptive, change in the opposing component of the system (Table 9-4). The adaptive response involves the component opposite the one disturbed and returns the pH of the system toward but not completely to normal. Overcompensation does not occur. For example, metabolic acidosis is accompanied by a secondary or adaptive respiratory alkalosis. Respiratory acidosis is accompanied by a secondary or adaptive metabolic alkalosis.
Simple and mixed acid-base disorders
An acid-base disorder is said to be simple if it is limited to the primary disorder and the expected secondary, or adaptive, response. The magnitude of the expected responses is considered in detail in the chapters devoted to the primary acid-base disorders (see Chapters 10 and 11). A mixed acid-base disorder is one that is characterized by the presence of at least two separate primary acid-base abnormalities occurring in the same patient. A mixed acid-base disorder should be suspected whenever the secondary, or adaptive, response exceeds or falls short of that expected. In dogs, for example, the expected response to metabolic acidosis is a 0.7-mm Hg decrease in Pco2 for each 1.0-mEq/L decrement in plasma HCO3− concentration caused by metabolic acidosis (see Chapter 10 for more details).
Consider a patient with the following blood gas values: p. 7.40, [H+] = 40 nEq/L, [HCO3−] = 31 mEq/L, and Pco2 = 51 mm Hg. This patient is neither alkalemicnoracidemic because blood pH is 7.40; however, based on the Pco2 and [HCO3−], the patient is not normal. This patient has a mixed disorder characterized by metabolic alkalosis and respiratory acidosis. The two disorders have counterbalancing effects, resulting in a normal pH. Mixed acid-base disorders are considered in detail in Chapter 12.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree