35 Intestinal disease
Introduction
The National Research Council (NRC) recognizes the importance of both nutritional and behavioral aspects of equine nutrition with their recommendation that carers “efficiently supply dietary ingredients in amounts that will meet the horse’s nutrient needs, while still retaining the horse’s normal feeding behavior” (NRC 2007c). In the context of performance of many equestrian pursuits, there is, however, an essential incompatibility of these two goals. The requirement for increased energy to fuel performance has generally been met by provision of nutrient-dense diets for which the equine gastrointestinal tract is not well adapted and, furthermore, are associated with restriction of many aspects of normal feeding behavior. In addition to possible adverse consequences on horses’ psychological well-being, this imbalance has further negative influences on gastrointestinal health and function.
Diets and feeding behaviors
Dietary causes of intestinal diseases are largely considered to result from intolerance of challenges manifestly distinct from the pressures that shaped evolutionary development of the equine gastrointestinal tract. Although the earliest recognizable equid ancestors browsed on dicotyledonous plants and fruits 55 million years ago, there followed an unimaginably slow evolution into a grazing animal in response to the gradual expansion of grasslands and decline of woodland ecosystems during the Miocene period approximately 18 million years ago (Janis et al 2000). Study of modern feral equids confirms their continuing status as grazers with typically around 90% of dietary intake composed of grasses, sedges and rushes (McInnes & Vavra 1987, Stuska et al 2008). Indeed, grazing is the prime activity of feral horses, ponies and donkeys occupying between 12 and 20 hours per day (Canacoo & Avornyo 1998, Duncan 1980, Kuntz et al 2006, Pratt et al 1986, van Dierendonck et al 1996). Studies of domesticated grazing or hay-fed ponies indicate that voluntary feeding times are no different to values in feral animals and typically around 16 hours per day (Crowell-Davis et al 1985, Sweeting et al 1985), with the majority of forage consumed during the daytime and early evening (Husted et al 2009). Voluntary fasting does not extend longer than 3 to 5 hours in healthy horses (Ralston 1984) and ingestive behavior is significantly increased by social interaction and visibility of other horses (Houpt 1990, Sweeting et al 1985). Ambulatory activity is also an integral part of grazing behavior (Lamoot et al 2005, Ralston 1984). Dietary changes certainly occur in feral horses although most probably in a slow and gradual fashion over several weeks with changes in the seasons and weather influencing quality and quantity of ingested herbage (McInnes & Vavra 1987, Stuska et al 2008).
Along with domestication of the horse came a requirement for improved dietary quality to match the energy expenditure associated with the activities required of them. Although such changes probably began more than 5000 years ago, this is a brief period of time in evolutionary terms. Under modern management systems many horses are fed relatively energy-dense, high-carbohydrate, low-fiber and occasionally high-fat feeds in meals that are often consumed in a relatively short period of time with prolonged periods spent without access to food (Clarke et al 1990, Houpt 1990). When complete pelleted or concentrate diets are offered to horses only around 4 to 10 hours per day is spent feeding (Houpt 1982, Ralston et al 1979) with increased time spent on other behaviors such as wood chewing and coprophagy (Willard et al 1977). Furthermore, many differing choices and sources of preserved forage and concentrate feeds are available to the horse owner creating a greater likelihood of abrupt dietary changes.
Epidemiology and risk factors for intestinal disease
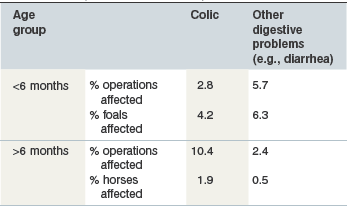
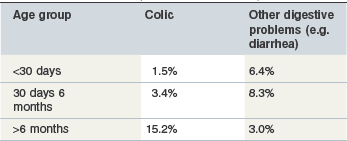
The commonest clinical signs of intestinal disease in horses comprise diarrhea and colic. Colic is generally considered to be the more important of the two presentations in terms of prevalence and cause of death in adult horses and the reverse is true in foals (USDA 2006, Tables 35-1 and 35-2). When horses present with diarrhea or colic in equine practice, a precise cause of disease is frequently not identified (Cohen et al 1999, Frederick et al 2009, Kaneene et al 1997, Love et al 1992, Mair et al 1990, Tinker et al 1997). Nevertheless epidemiologic studies have been helpful in highlighting several possible dietary associations with intestinal diseases.
Nutritional risk factors for diarrhea
Although dietary causes of diarrhea are commonly suspected there has been very little investigation of such with far greater research interest in infectious causes (Feary & Hassel 2006). Nutritional factors did not feature in either of two large studies of diarrhoea in adult horses (Love et al 1992, Mair et al 1990) although a specific cause is not identified in many cases in both foals and adults (Frederick et al 2009, Love et al 1992, Mair et al 1990). Nevertheless, several dietary factors are known to be capable of inducing diarrhea such as certain plants (e.g., acorns, blue-green algae, castor beans, heather) and other toxins (e.g., arsenic, selenium, raw linseed oil, propylene glycol) although none are seen commonly or well described (Cohen 2002). Dietary changes such as increased cereal feeding and increased grazing are anecdotally recognized as causes of diarrhea and consistent with this the administration of high doses of grain starch and/or oligofructose consistently induces diarrhea in horses (Rowe et al 1994, van Eps & Pollitt 2006).
Nutritional risk factors for colic
In contrast to diarrhea, far more epidemiologic investigation of colic has been published, perhaps as a result of its frequently distressing presentation and large economic impact (Traub-Dargatz et al 2001). Aspects of nutrition have featured heavily in such studies (Archer & Proudman 2006). Although epidemiologic studies of specific types of colic have been performed, many have combined all colic cases regardless of diagnosis. The fact that many studies may be unwittingly investigating many different underlying disease processes in various different parts of the gastrointestinal tract, with varying and quite possibly opposite causation, makes it remarkable that significant risk factors can be found. Nevertheless, many nutrition-related risk factors have indeed been identified for equine colic suggesting the existence of common and important pathophysiologic processes culminating in many different painful intestinal diseases. The following describes diet-related risk factors that have been associated with colic in general and also with specific types of colic.
Risk-factors for colic in general
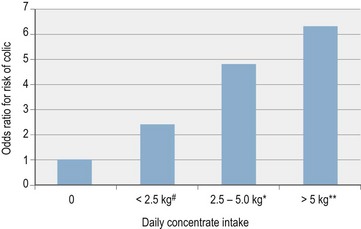
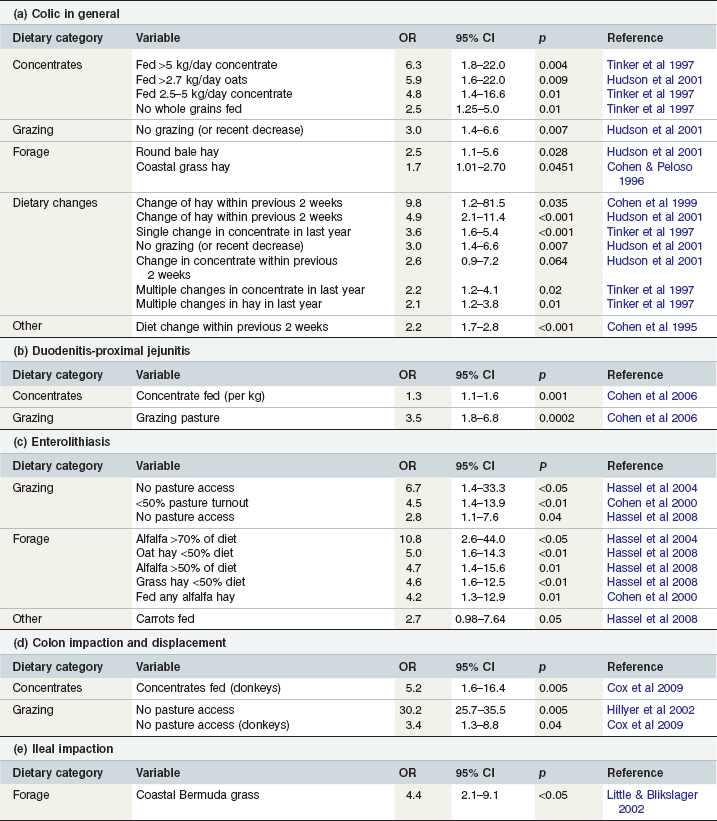
Providing horses with high-starch meals is a common but unnatural feeding practice and several studies have confirmed that the risk of colic significantly increases with higher levels of cereal or concentrate feeding. Such feeding practices are frequently associated with further potential colic risk-factors such as suboptimal forage intake, high levels of exercise, particular breeds of horses and perhaps restricted turnout although multivariate analysis has been applied in several studies to specifically identify causal factors (Fig. 35.1, Table 35-3a) (Hudson et al 2001, Kaya et al 2009, Tinker et al 1997). The consumption of 2.5–5.0 kg of concentrates daily was shown to increase the risk of suffering colic by almost 5 times in one study (Tinker et al 1997), whilst a separate study found a similar quantity of oats to be associated with almost 6 times more colic episodes (Hudson et al 2001). Higher levels of concentrate feeding were even more hazardous with horses fed >5 kg concentrate daily having a greater than 6 times increased risk of colic (Tinker et al 1997). Studies of racing Thoroughbreds and Standardbreds reported a mean concentrate intake of 7 to 8 kg/day (up to 13.2 kg/day) (Richards et al 2006, Southwood et al 1993) indicating that an increased incidence of colic is expected as an occupational hazard of competitive racing. It has been suggested that pelleted-, extruded- or sweet-feeds may pose a greater risk than whole grains (Morris et al 1989, Tinker et al 1997) although this is disputed by other studies (Cohen et al 1999, Kaya et al 2009, Little & Blikslager 2002).
Table 35-3 Nutritional and Diet-Related Factors Found to Increase the Risk of Colic in Multivariate Analyses
* Combination of grass cutting and cograzing ruminants may nullify risk reduction.
Free access to grazing is intuitively beneficial to intestinal health as this both mimics a natural diet and facilitates normal feeding behaviors and associated activities such as gentle exercise and social interaction. Pasture turnout has generally been found to be associated with a reduced risk of colic in comparison to stable confinement. Hudson et al (2001) found that horses that were fully stabled or had a recent reduction in grazing were three times as likely to have colic as those at pasture full time even after controlling for many other associated variables (Table 35-3a), although some studies have found no association between pasture access and colic risk (Cohen et al 1995, Cohen & Peloso 1996, Kaya et al 2009).
When grazing is restricted then preserved forages become the principal supply of fermentable carbohydrate. Some studies have shown no association between the type of hay fed and colic risk (Cohen et al 1995), whereas others have found the choice of hay to have a significant influence on colic (Cohen et al 1999). Feeding coastal grass hay or poor quality hay fed from round bales has been found to increase the risk of colic in general (Table 35-3a) (Cohen & Peloso 1996, Hudson et al 2001). In addition to nutritional quality, the hygienic quality of hay is also a significant consideration. In one study, almost 30% of hays fed to horses that developed colic were of low hygienic quality compared with only 4% of samples fed to control horses (p = 0.027, Kaya et al 2009).
Horses appear best adapted to a slow and continual intake of a diet of even quality and dietary changes appear to be poorly tolerated by the equine intestinal tract. Not all studies have confirmed any association between dietary change and colic (Cohen & Peloso 1996, Kaya et al 2009) although this does appear to be among the strongest and most consistently reported risk factors. Events such as changing the batch or type of hay or concentrate, changing stabling conditions, changing the quantity or frequency of feeding and erring from usual feeding times all significantly increase colic risk (Cohen et al 1995, Cohen & Peloso 1996, Tinker et al 1997) and it has generally been found that a recent change in hay or forage is more harmful than a recent change in grain or concentrate (Table 35-3a) (Cohen et al 1999, Hillyer et al 2002, Hudson et al 2001, Little & Blikslager 2002). In a study of horses with chronic intermittent colic, Cohen & Peloso (1996) found diet changes within the 2 week period prior to the colic to be a significant risk factor. After examining many possible aspects and interactions of dietary change, Cohen et al (1999) found that a change of hay in the previous 2 weeks increased the risk of colic by almost 10-fold and was the strongest diet-related risk factor in that study (Table 35-3a). In a separate study, Hudson et al (2001) found a recent dietary change to be associated with a significantly increased risk of colic although a change in hay posed almost double the risk of a concentrate change (Table 35-3a).
Risk factors for duodenitis-proximal jejunitis
Although concentrate feeding may increase the risk of colic in general (Tinker et al 1997), the association between concentrate feeding and duodenitis-proximal jejunitis (DPJ) appears to be especially strong (Table 35-3b). In one study, horses with DPJ were receiving a mean concentrate ration of 4.1 kg/day which was significantly greater than the 2.7 kg/day fed to other colic types and lame controls (Cohen et al 2006). In contrast to many other forms of colic, pasture access also represents a strong risk factor for DPJ (Table 35-3b) (Cohen et al 2006). A possible protective effect of feeding Bermuda grass hay against DPJ has been found although this might actually have resulted from other associated variables such as decreased grazing and increased concentrates (Cohen et al 2006, Morris et al 1989). In another study mixed legume-grass hay was more likely to be associated with DPJ cases than other forms of colic (Morris et al 1989).
Risk factors for enterolithiasis
Feeding alfalfa hay represents the strongest risk factor for colon obstruction with enteroliths (Table 35-3c) (Cohen et al 2000, Hassel et al 2004, 2008, Morris et al 1989). In contrast, provision of oat and grass hays has been shown to significantly reduce the risk of enterolith formation even after controlling for reduced alfalfa feeding (Table 35-3c) (Hassel et al 2008). A reduced incidence of enterolithiasis in horses fed Bermuda grass hay has also been suggested (Morris et al 1989). Similarly grazing appears to offer significant protection against enterolithiasis even after controlling for reduced alfalfa consumption (Table 35-3c) (Cohen et al 2000, Hassel et al 2004, 2008). A possible beneficial effect of mineral/vitamin supplementation was also reported for enterolithiasis although this did not reach statistical significance when controlled for other confounding factors (Hassel et al 2008). In contrast, feeding carrots was found to increase the risk of enterolithiasis even in multivariate analysis (Table 35-3c) (Hassel et al 2008).
Risk factors for intestinal impactions
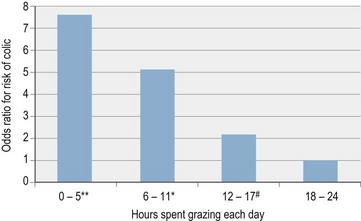
Grazing is a strong protective factor against development of colon impactions and displacements in horses and against colon impactions in donkeys (Table 35-3d, Fig. 35.2) (Cox et al 2009, Dabareiner & White 1995, Hillyer et al 2002). In one large study, horses with colon impactions and displacements were found to be receiving higher concentrate rations than controls although this appeared to be confounded by other factors such as reduced grazing (Hillyer et al 2002). However, in a recent study of colon impactions in donkeys, concentrate feeding remained significantly associated with increased risk of colon impactions even after controlling for other factors such as dental disease, weight loss and reduced grazing (Cox et al 2009).
Feeding coastal Bermuda grass hay compared with timothy, alfalfa, orchard grass or oat hays was the strongest risk factor for ileal impactions in one study (Table 35-3e) (Little & Blikslager 2002) and 11/48 (23%) cases had first received this hay within the previous 3 weeks (Little & Blikslager 2002). Although forage feeding is also very strongly associated with colon impaction and displacements in horses, this may be an indirect effect due to other variables associated with forage such as a reduction in exercise or less grazing time (Hillyer et al 2002). Interestingly, straw feeding in donkeys was not a risk factor for colon impactions and even appeared protective in univariable analysis (Cox et al 2009).
Risk factors for equine grass sickness
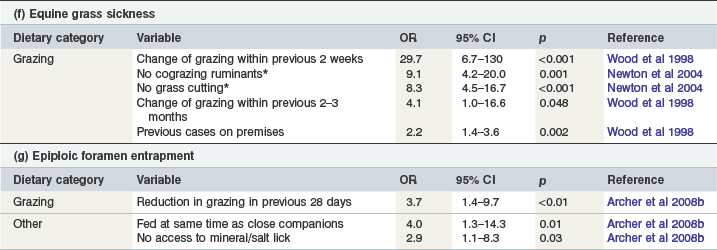
Aspects of grazing are strongly associated with equine grass sickness (EGS), a condition seen almost invariably in grazing horses (Table 35-3f) (McCarthy et al 2001, Wylie & Proudman 2009). Increased risks of EGS in certain premises and fields have also been demonstrated (Wood et al 1998). A recent change in grazing is recognized as a very strong risk factor for EGS (Doxey et al 1991, Wood et al 1998) and although the risk of EGS is greatest within 2 weeks of a change of grazing, the increased risk of disease is still significant as long as 2–3 months later (Table 35-3f) (Wood et al 1998).
Pasture management practices that significantly reduce the risk of EGS on previously affected premises include pasture cutting, manual feces removal and cograzing with ruminants (Table 35-3f) (Newton et al 2004). However, there appeared to be complex interactions between these practices such that the protective effect of manual feces collection or ruminant cograzing was reduced when combined with pasture cutting (Newton et al 2004). In contrast domestic birds sharing the pasture and use of mechanical paddock sweepers for removal of feces appear to increase the risk of EGS, especially on cut pastures (Newton et al 2004).
Risk factors for epiploic foramen entrapment
Although horses with epiploic foramen entrapment (EFE) spend less time grazing and more time stabled than control horses this may be an indirect relationship related to other factors such as dietary change and increased crib-biting/windsucking behavior in stabled horses (Archer et al 2008a,b). Horses experiencing a reduction in grazing within the previous 28 days were found to have an increased risk of suffering EFE even after controlling for other strong risk factors such as crib biting/windsucking (Table 35-3g) (Archer et al 2008b), although reduced grazing was not identified as a significant risk factor for EFE in a larger international study (Archer et al 2008a). The risk of EFE is reduced by feeding horses at different times from their close associates and also by offering horses mineral or salt licks (Table 35-3g) (Archer et al 2008b).
Risk factors for other types of colic
Along with EGS and DPJ, sand impaction is one of the few types of colic associated with grazing activity although clearly this is unlikely to be related to grass consumption per se (Ragle et al 1989). In addition to its strong association with enterolithiasis, alfalfa hay also represents the strongest risk factor for colic due to blister beetle (cantharidin) toxicity (Helman & Edwards 1997) but appears to decrease the risk of colic due to small intestinal strangulations (Morris et al 1989).
Pathophysiology of nutritional colic and diarrhea
The anatomy and physiology of the equine gastrointestinal tract implies strong adaptation to hindgut fermentation of structural fiber to provide a large supply of energy-rich short chain fatty acids (SCFAs) (Al Jassim & Andrews 2009, NRC 2007a). Starch and sugar intake was likely to be very low in the diet for which horses have evolved, and fructans far more limited than in modern cultivated pasture grasses. Analysis of a typical feral equine diet indeed reveals a very high proportion of cellulose and lignins (e.g., acid detergent fiber [ADF] 50% dry matter [DM]) and relatively low crude protein (e.g. 6% DM) and digestible energy (DE) (e.g. 7 MJ/kg DM) (McInnes & Vavra 1987, Stuska et al 2008). As horses’ appetites for forage is limited typically to approximately 2% bodyweight as DM daily, this suggests that their GI tracts are designed for an intake of around 140 kJ DE/kg bodyweight, coinciding with current estimates of equine maintenance energy requirements (127 to 152 kJ DE/kg, NRC 2007b). Consistent with this evolutionary dietary perspective there appears to be a significant disparity between small and large intestinal function in the horse. The equine small intestine is relatively deficient in alpha-amylase (Dyer et al 2002, Richards et al 2004) with a consequent poor capacity for the digestion of starch (Cuddeford 2000, Harris & Arkell 2005, Hintz 2000). In contrast, the fermentative efficiency of the equine large bowel is remarkable and dependent on several fibrolytic bacterial groups including Clostridium, Fibrobacter and Spirochaetaceae. These bacteria slowly ferment structural carbohydrates to SCFAs, primarily comprising acetate, propionate and butyrate that contribute the major source of energy to forage-fed horses (Al Jassim & Andrews 2009, Daly et al 2001, NRC 2007a). Stability and support of these normal and vital fermentative processes appear key to maintaining intestinal health.
In stark contrast to the high-fiber and limited caloric content of the typical feral diet, the nutritional demands of modern equine activities may require an intake of as much as 290 kJ DE/kg bodyweight (NRC 2007b) which would therefore require a ration energy density of approximately 10 to 15 MJ DE/kg dry matter (DM) even if DM intake increased substantially above 2% bodyweight. The traditional approach to increasing ration energy density has been by increasing provision of sugars and starches although vegetable oils have become more popular in recent years.
Among the most important causal factors to consider in the association between diet and colic or diarrhea is starch arriving in the hindgut after having overwhelmed small intestinal digestion (Fig. 35.3). High-cereal diets are likely to result in significant delivery of starch to the cecum and, additionally, small intestinal transit may be hastened by the more voluminous chyme associated with high starch feeds, further limiting prececal digestibility and increasing hindgut delivery of starch (Clarke et al 1990, Drogoul et al 2001, Metayer et al 2004). In addition to the large population of fibrolytic anaerobes in the hindgut, there is a smaller population of acidophilic, saccharolytic species, including Bacillus, Lactobacillus, Streptococcus and Mitsuokella that rapidly hydrolyze starch that has escaped small intestinal digestion, producing lactate and propionate (Al Jassim & Andrews 2009, Hoffman et al 2001, Milinovich et al 2008, Shirazi-Beechey 2008). When horses eat meals rich in starch, the arrival in the hindgut of such a rapidly fermentable carbohydrate initially increases the rate of bacterial multiplication and favors the growth of acidophilic bacteria that generate lactate and result in a fall in pH from the normal 6.7–7.0 to as low as 6.0. This acidification has further effects on bacterial populations including a reduction in fiber-fermenting species, decreased fiber digestibility and decreased SCFA absorption by the colon (Shirazi-Beechey 2008). Consequently further alterations in SCFA profiles occur including reduced acetate, increased proprionate and further increased lactate and decreased pH. The normally highly exclusive intestinal mucosal barrier may be disrupted by low pH leading to systemic absorption of bacterial lipopolysaccharide, exotoxins and vasoactive amines (Bailey et al 2003, Geor & Harris 2007) with subsequent effects on perfusion, pain and intestinal motility. Diarrhea, impaction, inflammation, dysmotility, distension with gas and froth, and subsequent colonic displacement and volvulus are all potential consequences (Clarke et al 1990, de Fombelle et al 2001, Drogoul et al 2001, Hussein et al 2004, Julliand et al 2001, Lopes et al 2004, Potter et al 1992). Fecal pH was less than 6.2 in more than a quarter of cereal-fed racehorses in one study (Richards et al 2006) consistent with probable adverse cecocolonic health and function. de Fombelle et al (2001) found that changing the diet from hay only to 70% hay and 30% rolled barley lead to significant alterations in hindgut bacterial flora and VFA concentrations with the potential for provoking colic. These abnormal patterns of increased colonic lactate and decreased fibrolysis have been confirmed within the large intestinal contents of horses with colic (Shirazi-Beechey 2008
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree