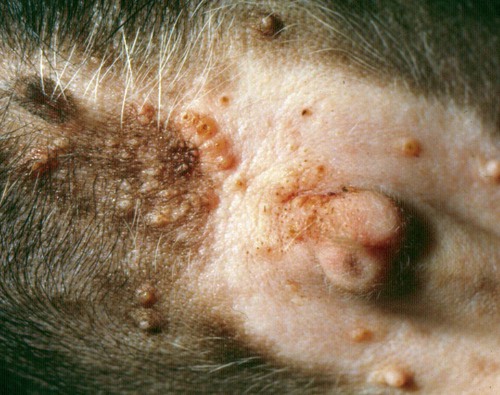
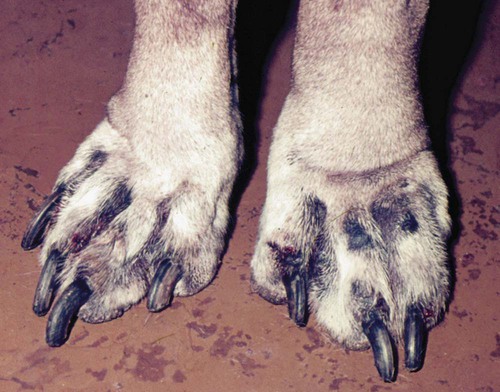
Pyoderma is defined as a pyogenic or pus-producing bacterial infection of the skin. The diversity of clinical syndromes seen with canine pyoderma is enormous, varying from minor annoyances to disease with life-threatening potential.* Pyoderma may affect the surface, creating inflammation without the invasion of living tissue; be superficial, involving the epidermis and intact hair follicle units; or invade more deeply, compromising the dermis and subjacent fatty tissue. This tremendous diversity and pleomorphism are responsible for diagnostic and management difficulties. Misdiagnosis also may result from the continuum of clinical characteristics and severity of the pyoderma, among individual dogs, different breeds, at different anatomic sites, and between acute and chronic disease. The presence of pus cannot be used as a defining diagnostic criterion because accumulations of dead neutrophils, of which pus is composed, may not be visible grossly. The aging and rupture of pustules lead to crusted papules that are not as diagnostic as pustules, because papules can result from many different inflammatory processes. In addition, accumulations of pus in the mid-dermis in deep pyoderma may not be visually obvious. Globally, pyoderma remains one of the most common causes of canine skin disease. Pyoderma was second only to flea allergy dermatitis in frequency of diagnosis in a study from North American veterinary colleges.89,178 An additional epidemiologic study performed in a relatively flea-free environment in Canada ranked bacterial folliculitis and furunculosis first among all canine skin diseases, comprising more than 25% of the dermatology caseload.177 Conversely, pyoderma is a relatively uncommon cause of skin disease in cats, other domestic animals, and humans. Bacterial skin disease in the cat is uncommon, with the exception of subcutaneous bite wound abscesses (see Chapter 51). The reasons for the markedly elevated frequency of bacterial skin disease in the dog in comparison with other mammalian species are not known. Various host factors that may result in enhanced susceptibility include the comparatively thin, compact canine stratum corneum; the relative paucity of intercellular lipids in the canine stratum corneum; the lack of a lipid-squamous epithelial plug in the entrance of canine hair follicles; and the relatively high pH of canine skin.89,113,126,176 Skin microbial flora is composed of resident and transient bacteria. Resident bacteria are harmless commensals that live on the skin surface and in hair follicles and maintain a static, consistent population. Transient bacteria usually cannot compete long term with the established resident flora but may seed the skin from mucous membranes (especially the nares and perianal region). The total number of resident bacteria residing on normal canine skin is not large and may comprise fewer than 350 organisms per square centimeter.89 Studies examining the bacterial flora of normal dogs have documented aerobic organisms, including Micrococcus species, β-hemolytic streptococci, and Acinetobacter species, and anaerobic organisms, including Clostridium perfringens and Propionibacterium acnes.* The most common canine cutaneous bacterial pathogen, formerly identified as Staphylococcus intermedius, is in fact a closely related species, Staphylococcus pseudintermedius.50,167 Although many prior published works and cited references use the former terminology, for clarity and accuracy the correct term, S. pseudintermedius, is substituted throughout this chapter (see Chapter 34). The role of S. pseudintermedius on canine skin is better understood.† This facultative pathogen is a resident primarily in the nares and perianal region, a transient, restricted, local colonist on clinically healthy canine skin, and a contaminant on canine hair. Seeding of the skin from the perianal region and nares probably occurs during both normal grooming and excessive licking by pruritic and especially allergic dogs.3–5,89,111 Considerably less frequently than S. pseudintermedius, Staphylococcus aureus and Staphylococcus schleiferi can cause skin infection in dogs.65,71,130,139 Most likely the novel awareness of these two species is explained because they are grouped generically with S. pseudintermedius or simply not speciated in the past. Methicillin-resistant (MR) staphylococci (MRS) and especially MR S. aureus (MRSA) have been a “hot button” issue in human medicine for more than a decade. The identification of MRS and especially MRSA in dogs, cats, and horses also has focused scrutiny on their prevalence in animals.110 Carriage and infection with MR S. pseudintermedius (MRSP), MR S. schleiferi, and MRSA are being identified globally in dogs and cats with seemingly increased prevalence. It is important to emphasize that MRS do not possess any greater virulence than methicillin-sensitive staphylococci. However, effective treatment is more problematic. Because of escalating concern for MRS and multidrug-resistant staphylococci, bacterial culture and susceptibility testing are being performed much more frequently than in the past. More frequent culture has led to heightened awareness that other species of staphylococci may cause pyoderma in animals. Pure cultures of S. pseudintermedius are grown from most pustules or draining tracts in dogs with pyoderma. Infection with S. aureus may actually be more common in cats than in dogs. Further details on the ecology and epidemiology of staphylococcus in animals and humans are found elsewhere in this book (see Chapter 34). Pathogenicity of S. aureus in humans can correlate with virulence factors such as adhesins (protein A); secreted enzymes such as proteases, hyaluronidase, and lipases; clumping factors; various other binding proteins; leukocidin; and toxins. S. pseudintermedius exfoliative toxin has been identified, but a role in disease has not been proven. When potential virulence factors have been examined comparing S. pseudintermedius isolates from normal dogs and dogs with pyoderma, clear differences in toxin profiles, gel electrophoresis of exoproteins, and immunoblotting of concentrated extracellular proteins were not noted.2,4,24,64,67 Production of exotoxins does not appear to play a role in the pathogenicity of S. pseudintermedius for canine skin.24 Evidence does not support virulence factors as the cause of differences in canine susceptibility or severity of infection.24,32,80,89,90 Data suggest that host factors rather than virulence factors appear to be more important in determining susceptibility, severity, and clinical outcome in canine staphylococcal pyoderma.* Secondary gram-negative invaders such as Proteus species, Pseudomonas species, or Escherichia coli may be isolated in conjunction with S. pseudintermedius, usually from deep pyoderma. However, if gram-negative bacteria are isolated from pyoderma without the concomitant isolation of gram-positive staphylococcus, the technique used and the results obtained should be questioned, because canine pyoderma caused by gram-negative bacteria without staphylococcal co-infection is uncommon. Infection with S. pseudintermedius creates a tissue milieu that is more conducive to secondary invasion by gram-negative bacteria.88,89 The factors that promote the proliferation of S. pseudintermedius on skin leading to pyoderma are poorly understood. However, it is well established that dogs with other skin diseases are more likely to develop secondary pyoderma. Dogs with allergic skin diseases such as atopic dermatitis or defects in cornification exhibit a shift in the balance of bacterial species colonizing the skin such that coagulase-positive staphylococci can predominate.89 Clinically, this correlates with an increased frequency of pyoderma. Most public health concerns regarding dogs and cats and staphylococci are linked to MRSA. Colonization or infection in dogs or cats living in households with owners colonized with MRSA has been widely reported.* According to compilations by others,194 it is likely that human-to-animal transmission occurs as least as commonly, if not more often, than animal-to-human transmission (see Chapter 34). S. schleiferi also is of potential public health concern, because this organism can be equally pathogenic in either humans or animals. The zoonotic potential for MRSP in humans is substantially less, because S. pseudintermedius has low pathogenicity for humans and opportunistic infection by MRSP in humans is rare. Humans with a normally functioning immune system are not at great risk for skin infections caused by S. pseudintermedius. Dogs also harbor S. pseudintermedius in their oral cavities; up to 21% of dog-bite lesions in humans may be contaminated with this organism.184 (For an additional discussion see Staphylococcal Infections, Chapter 34, Bite Wound Infections, Chapter 51, and Zoonotic Infections of Medical Importance in Immunocompromised People, Chapter 99). In comparison with S. aureus, S. pseudintermedius does not possess the requisite virulence factors to be a potent pathogen. Consequently, most cases of canine pyoderma probably are associated with underlying disease or other host immune factors. Diseases such as allergies (atopic dermatitis, food allergy, flea-allergy dermatitis), ectoparasitism, cornification defects (seborrhea), hereditary skin diseases (genodermatoses)—especially those affecting hair follicles—and endocrinopathies such as hypothyroidism and Cushing’s disease frequently predispose dogs to secondary pyoderma.46,89,103,175,176 Pyoderma secondary to allergic diseases and cornification defects are best documented. More broadly, pruritus from any underlying disease, cutaneous inflammation from any cause, injudicious excessive use of glucocorticoids (iatrogenic hyperglucocorticoidism), and poor grooming in long-coated dogs all contribute to the likelihood of secondary pyoderma. Superficial infection of the hair follicle, or folliculitis, is the most common canine pyoderma. Follicular defects, dysplasia, obstruction, atrophy, inflammation, or degeneration predispose to folliculitis. After pyoderma is initiated, immunologic incompetence, coexisting skin disease, pruritus, inflammation, scar tissue formation, and improper initial therapy are negative prognostic factors.89 The initiation of staphylococcal pyoderma requires colonization (bacterial overgrowth) and frequently invasion of host tissues in addition to evasion of host immunity. Host defense mechanisms mobilized to prevent bacterial invasion include immunologic and nonimmunologic processes. Nonimmunologic mechanisms include the desquamation of the stratum corneum (surface and follicular), the lipid intercellular barrier, epithelial proliferation in response to injury, and the antibacterial effect of inorganic salts found in sebum and sweat. Additionally, competition among resident bacteria is a nonimmunologic, “nonhost” defense mechanism. Immunologic host defense mechanisms of the skin include proteins within the intercellular matrix; immunoglobulins within the basement membrane zone; and immunologically active cells such as Langerhans cells, dermal dendrocytes, lymphocytes, mast cells, and venular endothelial cells present in either the epidermis or dermis.89 Host immunologic response may be deleterious as well as beneficial. Some dogs with chronic or recurrent pyoderma exhibit depression of lymphocyte transformation testing. Exceptionally potent bacterial antigens, termed superantigens, may explain the troublesome nature of pyoderma secondary to canine atopic dermatitis and the marked inflammation and pruritus seen in some canine pyoderma.89 Bacterial hypersensitivity has long been theorized as a complicating factor in recurrent canine pyoderma. The potential importance of bacterial hypersensitivity has been underscored by work indicating that mast cell degranulation can initiate enhanced epidermal permeability to bacterial antigens in atopic dogs.89,125 Several studies have verified an association between anti-staphylococcal antibodies and various subgroups of canine pyoderma.89,140 Classification based on depth of bacterial involvement is most useful clinically because it provides information on diagnosis, likelihood of underlying disease, prognosis, required duration of therapy, and response to therapy. In general, the deeper the infection, the more likely that specific underlying triggering causes are present. Deeper infections also require that the clinician be more aggressive diagnostically and therapeutically. Using depth of bacterial infection, canine pyoderma can be described as surface, superficial, or deep (Box 84-1).89 Surface pyodermas consist of inflammatory processes in the skin without strong evidence of direct bacterial invasion of living tissue. Bacterial involvement probably is secondary to factors that encourage surface bacterial overgrowth. Pyotraumatic dermatitis (acute moist dermatitis, hot spots), intertrigo (skin-fold pyoderma), mucocutaneous pyoderma, and surface bacterial overgrowth are classified as surface pyoderma. Pyotraumatic dermatitis usually develops secondary to flea-allergy dermatitis. Intertrigo occurs in skin folds secondary to breed-characteristic anatomic defects and is seen in conjunction with friction, poor drainage, and maceration. In mucocutaneous pyoderma, bacterial involvement may become deeper with chronicity. Mucocutaneous pyoderma is a surface disease of unknown cause that predominantly involves the lips and perioral skin but may involve other mucocutaneous sites such as the anus.89,92 Pyotraumatic dermatitis and intertrigo rarely are diagnostic or therapeutic challenges. Conversely, mucocutaneous pyoderma and bacterial overgrowth can present both diagnostic and therapeutic challenges.154 Superficial pyodermas are the most common canine bacterial skin diseases. Impetigo is characterized by nonfollicular, intraepidermal pustules involving the superficial layers of the epidermis (Fig. 84-1). Superficial folliculitis affecting the ostial portion of the hair follicle is the most common canine pyoderma. Impetigo and superficial folliculitis may be diagnostically challenging because pustules rupture readily, giving rise to considerably less diagnostic crusted papules. A third clinical subset of superficial pyoderma, termed superficial spreading pyoderma, is characterized by centrifugally expanding inflammation with characteristic peripheral peeling epidermal collarettes. Superficial spreading pyoderma may be seen alone or in conjunction with superficial folliculitis. Deep pyodermas proceed deeper in the hair follicle with or without follicular rupture. Factors that allow infection to proceed from superficial to deep folliculitis are not understood. Deep folliculitis can lead to follicular rupture (furunculosis) with a granulomatous foreign body tissue response (Fig. 84-2). Interconnecting furunculosis involving the interstitium between hair follicles, the dermis, and subcutis is termed cellulitis. Cellulitis commonly initiates sepsis. Deep pyoderma is much less common than superficial pyoderma. Although diagnosis of deep pyoderma usually is not difficult, therapy often is problematic. Cellulitis results in follicular obliteration. Follicular rupture in deep pyoderma leads to nodule formation and draining fistulous tracts (see Fig. 84-2). Dermal hemorrhage resulting from follicular rupture may result in hemorrhagic bullae visible as dark bluish regions in the dermis. Many skin diseases can mimic pyoderma. Differential diagnoses are listed in an approximate order of importance in Box 84-2.89,175 Various diagnostic procedures are helpful in diagnosing pyoderma and determining the presence of underlying diseases or other predisposing factors. Skin scrapings, cytologic examination of pustules or the skin surface, and skin biopsy are the most useful diagnostic procedures for the evaluation of suspected pyoderma.89 Bacterial culture, identification and antibacterial susceptibility testing are now recommended more frequently as the prevalence of antibacterial drug resistance has increased dramatically in many regions of the world during the past 5 years. Reliable diagnostic tests to determine immunocompetence in the dog are not available.45,89 Gross information can be derived from a complete blood (cell) count (CBC) and serum protein electrophoresis. An absolute neutrophilia with a lymphocyte count of at least 1000 to 1500 cells/mL should be observed in normal dogs with ongoing or recurrent pyoderma. A broad-based elevation in the serum protein electrophoretic pattern in the β and γ ranges should be present.88,89 Assays such as in vitro lymphocyte stimulation and bactericidal tests remain research tools because of their expense, lack of reproducibility and lack of availability. The lack of ability to correct any defects that are documented further detracts from the clinical usefulness of these tests.46,176 Pyoderma, especially when deep, is associated with a high prevalence of circulating immune complexes. Dogs with chronic deep pyoderma are more likely to be proteinuric, with a predominance of albuminuria, than dogs with superficial pyoderma.13 Proteinuria is suspected to be a consequence of circulating immune complexes depositing in glomerular microcapillaries. An ideal empiric antibacterial should have a narrow spectrum of activity, minimal side effects, and reasonable cost and should have been shown to be effective in the management of canine pyoderma. Little clinical evidence exists that bactericidal agents are more effective than bacteriostatic agents in the management of uncomplicated superficial pyoderma. Bactericidal antibacterials are recommended if hair follicle defects are present, in most deep pyoderma cases, and when immunosuppression is suspected or confirmed. If culture is performed, pustules or fistulous tracts should be recultured if gram-positive staphylococci are not isolated as the primary pathogen. If multiple isolates are not sensitive to a single oral antibacterial, an antibacterial effective against staphylococci should be instituted because staphylococci create a tissue milieu favorable to the replication of secondary bacterial invaders. Results of culture and susceptibility studies and detailed information on individual antibacterials are discussed in greater detail in the Drug Formulary in the Appendix.89,175,175 Antibacterials useful in the management of canine pyoderma are listed in Table 84-1. Penicillin, ampicillin, amoxicillin, and tetracycline are poor choices for the treatment of canine pyoderma. Previous and regional use may alter antibacterial susceptibility.59,62,86,89,116 Not surprisingly, resistant S. pseudintermedius and gram-negative isolates are seen more commonly in referral practices than in general practice, and resistant bacterial populations are identified most frequently in deep pyoderma.86,89,89 Clinical trials have shown multiple antibacterials to be effective in managing canine pyoderma. Erythromycin, tylosin, lincomycin, clindamycin, chloramphenicol, trimethoprim and ormetoprim-potentiated sulfonamides, oxacillin, cephalexin, cefadroxil, cefpodoxime, quinolones, and amoxicillin-clavulanate have been successful in the treatment of various forms of canine pyoderma.* Preferred narrow-spectrum antibacterials still include erythromycin, lincomycin, and oxacillin, and preferred broad-spectrum antibacterials include cephalexin, cefadroxil, cefpodoxime, trimethoprim and ormetroprim-potentiated sulfonamides, and quinolones such as enrofloxacin and marbofloxacin. TABLE 84-1 Oral Antibacterial Drugs Useful for Treating Canine Pyoderma GI, Gastrointestinal; MRS, methicillin-resistant staphylococci. aFor additional information on listed drugs, see the Drug Formulary in the Appendix. Treatment generally lasts for a minimum of 21 days. bGive dose twice daily on the first day. Table modified from Ref. 88. For many years, it had been predicted that antibacterial-resistant S. pseudintermedius would preclude the administration of many antibacterials common in dermatology. Similarities and differences in antibacterial susceptibility patterns published during the 2 decades before the late 1990s indicated very little change.89 Unfortunately, a rise in staphylococcal resistance has occurred over the past decade and more rapidly during that interval (see Chapter 34). Owner compliance using different dosing schedule regimens is not well studied in veterinary medicine. Some perceived differences in efficacy may correlate with differences in compliance. Compliance is more likely to be achieved with antibacterials requiring dosing only once or twice daily than with those that need to be given three times daily. Cefpodoxime, ormetoprim-potentiated sulfadimethoxine, and the quinolones are the only antibacterials useful in canine pyoderma that can be administered once daily.34 Cephalexin, cefadroxil, and lincomycin require twice-daily dosing. Other suggested antibacterials require three daily doses. Various tiered systems for antibacterial use have been popularized.46,89,103,175,176 Cephalexin, cefadroxil, erythromycin, lincomycin, clindamycin, and ormetoprim-potentiated sulfadimethoxine are useful for the management of uncomplicated, first-occurrence surface and superficial pyoderma. The advantages and disadvantages of these drugs are listed in Table 84-1. Trimethoprim-potentiated sulfonamides, chloramphenicol, veterinary quinolones, and amoxicillin-clavulanate are possible alternative candidates for use in pyoderma. However, the potential side effects of trimethoprim sulfonamides are of concern.102 Quinolones offer the advantages of once-daily dosing, excellent tissue penetration, activity against S. pseudintermedius and gram-negative secondary invaders.53,89,89 Once-daily dosing is recommended because the bactericidal effect is concentration rather than time dependent.93,137 Uptake of enrofloxacin by macrophages leads to potent tissue-penetrating and concentrating abilities.53,93 Antibacterial gels, creams, and ointments may be applied in the treatment of limited areas of skin. Cost, messiness, and time required for application limit their usefulness. Benzoyl peroxide is available in a gel vehicle. Mupirocin is a potent antibacterial agent with superior penetrating ability formulated for skin but not mucosal surfaces. Mupirocin should not be used when absorption of large amounts of the polyethylene glycol vehicle is likely because of the potential for nephrotoxicity.89 Fusidic acid, a topically applied steroidal antibacterial not available in the United States, has activity against gram-positive bacteria, such as staphylococci.163 Immunomodulators can be either bacterial or nonbacterial preparations. Commercial bacterial preparations contain either killed Staphylococcus or Propionibacterium species as the antigen. Staphage Lysate (Delmont Laboratories, Swarthmore, PA) is the most common commercial bacterin used in North America and contains bacterial antigens of S. aureus isolated from humans. Staphage Lysate is the only product for which efficacy has been documented (approximately 40% of cases using 0.5 mL twice weekly48) by double-blinded, placebo-controlled studies. Autogenous bacterins occasionally are made from specific staphylococcal organisms isolated from a dog with pyoderma for use in that dog. Inactivation methodology is crucial because the process must kill the organism without disrupting antigenic determinants.
Integumentary Infections
Bacterial Infections of the Skin
Etiology and Pathogenesis
Normal Microflora of the Skin and Hair
Staphylococcus pseudintermedius and Other Canine Cutaneous Pathogens
Microbial Alterations with Skin Disease
Zoonotic Potential of Skin Pathogens
Susceptibility and Host Response to Infection
Classification of Pyoderma
Surface Pyoderma
Superficial Pyoderma
Deep Pyoderma
Clinical Findings
Secondary Skin Lesions
Diagnosis
Differential Diagnoses
Evaluation for Immunocompetence
Therapy
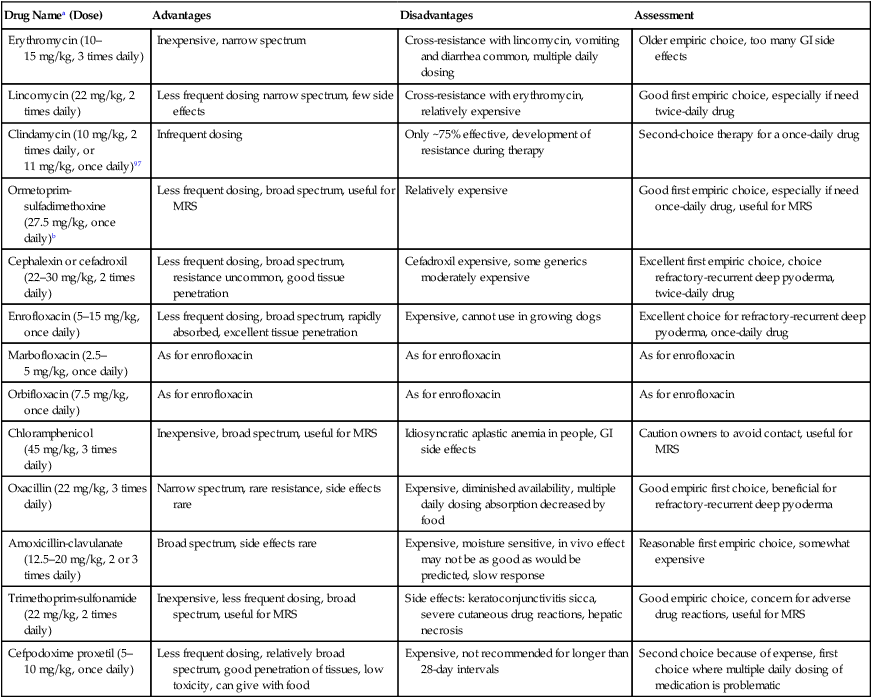
Antibacterial Therapy
Drug Namea (Dose)
Advantages
Disadvantages
Assessment
Erythromycin (10–15 mg/kg, 3 times daily)
Inexpensive, narrow spectrum
Cross-resistance with lincomycin, vomiting and diarrhea common, multiple daily dosing
Older empiric choice, too many GI side effects
Lincomycin (22 mg/kg, 2 times daily)
Less frequent dosing narrow spectrum, few side effects
Cross-resistance with erythromycin, relatively expensive
Good first empiric choice, especially if need twice-daily drug
Clindamycin (10 mg/kg, 2 times daily, or 11 mg/kg, once daily)97
Infrequent dosing
Only ~75% effective, development of resistance during therapy
Second-choice therapy for a once-daily drug
Ormetoprim-sulfadimethoxine (27.5 mg/kg, once daily)b
Less frequent dosing, broad spectrum, useful for MRS
Relatively expensive
Good first empiric choice, especially if need once-daily drug, useful for MRS
Cephalexin or cefadroxil (22–30 mg/kg, 2 times daily)
Less frequent dosing, broad spectrum, resistance uncommon, good tissue penetration
Cefadroxil expensive, some generics moderately expensive
Excellent first empiric choice, choice refractory-recurrent deep pyoderma, twice-daily drug
Enrofloxacin (5–15 mg/kg, once daily)
Less frequent dosing, broad spectrum, rapidly absorbed, excellent tissue penetration
Expensive, cannot use in growing dogs
Excellent choice for refractory-recurrent deep pyoderma, once-daily drug
Marbofloxacin (2.5–5 mg/kg, once daily)
As for enrofloxacin
As for enrofloxacin
As for enrofloxacin
Orbifloxacin (7.5 mg/kg, once daily)
As for enrofloxacin
As for enrofloxacin
As for enrofloxacin
Chloramphenicol (45 mg/kg, 3 times daily)
Inexpensive, broad spectrum, useful for MRS
Idiosyncratic aplastic anemia in people, GI side effects
Caution owners to avoid contact, useful for MRS
Oxacillin (22 mg/kg, 3 times daily)
Narrow spectrum, rare resistance, side effects rare
Expensive, diminished availability, multiple daily dosing absorption decreased by food
Good empiric first choice, beneficial for refractory-recurrent deep pyoderma
Amoxicillin-clavulanate (12.5–20 mg/kg, 2 or 3 times daily)
Broad spectrum, side effects rare
Expensive, moisture sensitive, in vivo effect may not be as good as would be predicted, slow response
Reasonable first empiric choice, somewhat expensive
Trimethoprim-sulfonamide (22 mg/kg, 2 times daily)
Inexpensive, less frequent dosing, broad spectrum, useful for MRS
Side effects: keratoconjunctivitis sicca, severe cutaneous drug reactions, hepatic necrosis
Good empiric choice, concern for adverse drug reactions, useful for MRS
Cefpodoxime proxetil (5–10 mg/kg, once daily)
Less frequent dosing, relatively broad spectrum, good penetration of tissues, low toxicity, can give with food
Expensive, not recommended for longer than 28-day intervals
Second choice because of expense, first choice where multiple daily dosing of medication is problematic
Topical Therapy
Immunomodulatory Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Integumentary Infections